Abstract
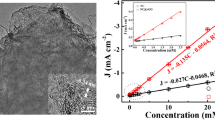
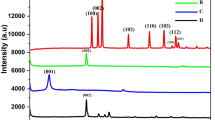
Porous carbon was prepared from a zeolitic imidazolate framework (type ZIF-8) by carbonization at 800 °C (Z-800). A hybrid material was then obtained by direct co-electrodeposition of Z-800 with graphene oxide (Z-800/rGO). Z-800 is N-doped with good electrical conductivity and displays electrocatalytic activity. Z-800 readily undergoes mass transfer and also prevents graphene to agglomerate during electroanalysis. The hybrid was placed on a glassy carbon electrode (GCE) to obtain an electrochemical sensor for chloramphenicol (CAP) detection. Under the optimized conditions, the response of the modified GCE (typically measured at a low potential of −0.07 V vs. Ag/AgCl) is linear in the 1 to 180 μM CAP concentration range with a 0.25 μM detection limit (S/N = 3). In our preception, the method has a wide scope in that it may be applied to the preparation of various kinds of other (doped) porous carbon/rGO composites for use in (bio)chemical sensors.

Schematic presentation of the preparation process of the materials and the electrochemical detection of chloramphenicol.






Similar content being viewed by others
References
Shalit I, Marks MI (1984) Chloramphenicol in the 1980s. Drugs 28:281–291. https://doi.org/10.2165/00003495-198428040-00001
Festing MFW, Diamani P, Turton JA (2001) Strain differences in haematological response to chloroamphenicol succinate in mice: implications for toxicological research. Food Chem Toxicol 39:375–383. https://doi.org/10.1016/S0278-6915(00)00149-6
Chen H, Chen H, Ying J, Huang J, Liao L (2009) Dispersive liquid–liquid microextraction followed by high-performance liquid chromatography as an efficient and sensitive technique for simultaneous determination of chloramphenicol and thiamphenicol in honey. Anal Chim Acta 632:80–85. https://doi.org/10.1016/j.aca.2008.10.068
Li P, Qiu Y, Cai H, Kong Y, Tang Y, Wang D, Xie M (2006) Simultaneous determination of chloramphenicol, thiamphenicol, and florfenicol residues in animal tissues by gas chromatography/mass spectrometry. Chin J Chromatogr 24:14–18. https://doi.org/10.1016/S1872-2059(06)60002-3
Rodziewicz L, Zawadzka I (2008) Rapid determination of chloramphenicol residues in milk powder by liquid chromatography-electrospray ionization tandem mass spectrometry. Talanta 75:846–850. https://doi.org/10.1016/j.talanta.2007.12.022
Gómez-Taylor B, Palomeque M, MateoJVG CJM (2006) Photoinduced chemiluminescence of pharmaceuticals. J Pharmaceut Biomed 41:347–357. https://doi.org/10.1016/j.jpba.2005.11.040
Yang G, Zhao F (2015) Electrochemical sensor for chloramphenicol based on novel multiwalled carbon nanotubes@molecularly imprinted polymer. Biosens Bioelectron 64:416–422. https://doi.org/10.1016/j.bios.2014.09.041
Zhou YL, Sui CJ, Yin HS, Wang Y, Wang MH, Ai SY (2018) Tungsten disulfide (WS2) nanosheet-based photoelectrochemical aptasensing of chloramphenicol. Microchim Acta 185:453–461. https://doi.org/10.1007/s00604-018-2970-8
Chen M, Gan N, Zhang HR, Yan ZD, Li TH, Chen YJ, Xu Q, Jiang QL (2016) Electrochemical simultaneous assay of chloramphenicol and PCB72 using magnetic and aptamer-modified quantum dot-encoded dendritic nanotracers for signal amplification. Microchim Acta 183:1099–1106. https://doi.org/10.1007/s00604-015-1695-1
Borowiec J, Wang R, Zhu L, Zhang J (2013) Synthesis of nitrogen-doped graphene nanosheets decorated with gold nanoparticles as an improved sensor for electrochemical determination of chloramphenicol. Electrochim Acta 99:138–144. https://doi.org/10.1016/j.electacta.2013.03.092
Kong FY, Chen TT, Wang JY, Fang HL, Fan DH, Wang W (2016) UV-assisted synthesis of tetrapods-like titanium nitride-reduced graphene oxide nano-hybrids for electrochemical determination of chloramphenicol. Sensor Actuat B-Chem 225:298–304. https://doi.org/10.1016/j.snb.2015.11.041
Yan ZD, Gan N, Li TH, Cao YT, Chen YJ (2016) A sensitive electrochemical aptasensor for multiplex antibiotics detection based on high-capacity magnetic hollow porous nanotracers coupling exonuclease-assisted cascade target recycling. Biosens Bioelectron 78:51–57. https://doi.org/10.1016/j.bios.2015.11.019
Lee J, Kim J, Hyeon T (2006) Recent progress in the synthesis of porous carbon materials. Adv Mater 18:2073–2094. https://doi.org/10.1002/adma.200501576
Banerjee R, Furukawa H, Britt D, Knobler C, O’Keeffe M, Yaghi OM (2009) Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J Am Chem Soc 131:3875–−3877 https://pubs.acs.org/doi/abs/10.1021/ja809459e
Ryoo R, Joo SH, Jun (1999) Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J Phys Chem B 103:7743–7746 https://pubs.acs.org/doi/abs/10.1021/jp991673a
Lu AH, Schüth F (2006) Nanocasting: a versatile strategy for creating nanostructured porous materials. Adv Mater 18:1793–1805. https://doi.org/10.1002/adma.200600148
Chaikittisilp W, Ariga K, Yamauchi Y (2013) A new family of carbon materials: synthesis of MOF-derived nanoporous carbons and their promising applications. J Mater Chem A 1:14–19 https://pubs.rsc.org/en/Content/ArticleLanding/2013/TA/C2TA00278G#!divAbstract
Kaneti YV, Tang J, Salunkhe RR, Jiang XC, Yu AB, Wu KC, Yamauchi Y (2017) Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks. Adv Mater 29(12). https://doi.org/10.1002/adma.201604898
Janiak C, Vieth JK (2010) MOFs, MILs and more: concepts, properties and applications for porous coordination networks (PCNs). J Cheminformatics 34:2366–2388 https://pubs.rsc.org/en/Content/ArticleLanding/2010/NJ/c0nj00275e#!divAbstract
Babarao R, Hu ZQ, Jiang JW (2007) Storage and separation of CO2 and CH4 in silicalite, C168 schwarzite, and IRMOF-1: a comparative study from Monte Carlo simulation. Langmuir 23:659–666 https://pubs.acs.org/doi/abs/10.1021/la062289p
Phan A, Doonan CJ, Uribe-romo FJ, Knobler CB, O’Keeffe M, Yaghi OM (2010) Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc Chem Res:58–67 https://pubs.acs.org/doi/abs/10.1021/ar900116g
Torad NL, Hu M, Kamachi Y, Takai K, Imura M, Naito M, Yamauchi Y (2013) Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem Commun 49:2521–2523 https://pubs.rsc.org/en/Content/ArticleLanding/2013/CC/c3cc38955c#!divAbstract
Chen LF, Lu Y, Lou XW (2017) Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ Sci 10:1777–1783 https://pubs.rsc.org/en/Content/ArticleLanding/2017/EE/C7EE00488E#!divAbstract
Zhong HX, Wang J, Zhang YW, Xu WL, Xing W, Xu D, Zhang YF, Zhang XB (2015) ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew Chem Int Edit 53:14235–14239. https://doi.org/10.1002/anie.201408990
Liu CB, Wang K, Luo SL, Tang YH, Chen LY (2011) Direct electrodeposition of graphene enabling the one-step synthesis of graphene-metal nanocomposite films. Small 7:1203–1206. https://doi.org/10.1002/smll.201002340
Pan Y, Liu YY, Zeng GF, Zhao L, Lai ZP (2011) Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem Commun 47:2071–2073 https://pubs.rsc.org/en/Content/ArticleLanding/2011/CC/c0cc05002d#!divAbstract
Xu JY, Xia JF, Zhang FF, Wang ZH (2017) An electrochemical sensor based on metal-organic framework-derived porous carbon with high degree of graphitization for electroanalysis of various substances. Electrochim Acta 251:71–80. https://doi.org/10.1016/j.electacta.2017.08.114
Hummers JWS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Zhang YX, Xu JY, Xia JF, Zhang FF, Wang ZH (2018) MOF-derived porous Ni2P/graphene composites with enhanced electrochemical properties for sensitive nonenzymatic glucose sensing. ACS Appl Mater Interfaces 10:39151–39160 https://pubs.acs.org/doi/10.1021/acsami.8b11867
Yang ZX, Xia YD, Sun XZ, Mokaya R (2006) Preparation and hydrogen storage properties of zeolite-templated carbon materials nanocast via chemical vapor deposition: effect of the zeolite template and nitrogen doping. J Phys Chem B 110:18424–18431 https://pubs.acs.org/doi/abs/10.1021/jp0639849
Zhang X, Zhang YC, Zhang JW (2016) A highly selective electrochemical sensor for chloramphenicol based on three-dimensional reduced graphene oxide architectures. Talanta 161:567–573. https://doi.org/10.1016/j.talanta.2016.09.013
Bai X, Qin CD, Huang X (2016) Voltammetric determination of chloramphenicol using a carbon fiber microelectrode modified with Fe3O4 nanoparticles. Microchim Acta 183:2973–2981. https://doi.org/10.1007/s00604-016-1945-x
Munawar A, Tahir MA, Shaheen A, Lieberzeit PA, Khan WS, Bajwa SZ (2018) Investigating nanohybrid material based on 3D CNTs@cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J Hazard Mater 342:96–106. https://doi.org/10.1016/j.jhazmat.2017.08.014
Codognoto L, Winter E, Doretto KM, Monteiro GB, Rath S (2010) Electroanalytical performance of self-assembled monolayer gold electrode for chloramphenicol determination. Microchim Acta 169:345–351. https://doi.org/10.1007/s00604-010-0339-8
Acknowledgements
We really appreciate the financial support from the National Natural Science Foundation of China (21645007 and 21475071), the Taishan Scholar Program of Shandong Province (No.ts201511027) and the Natural Science Foundation of Shandong (ZR2016BM21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 5.16 mb)
Rights and permissions
About this article
Cite this article
Yuan, Y., Xu, X., Xia, J. et al. A hybrid material composed of reduced graphene oxide and porous carbon prepared by carbonization of a zeolitic imidazolate framework (type ZIF-8) for voltammetric determination of chloramphenicol. Microchim Acta 186, 191 (2019). https://doi.org/10.1007/s00604-019-3298-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3298-8