Abstract
The authors describe the synthesis and characterization of 3-mercaptopropionylamidoxime functionalized gold nanoparticles (AuNPs) for visual detection of uranium (U) by cloud point extraction. The method is capable of quantifying U at the concentration limits set by the World Health Organization in drinking water i.e., 30.0 ng mL−1. The method is based on the gradual color change from red to blue that occurs as a result of the interaction between uranyl ion and the modified AuNPs leading to particle aggregation. Such analyte-triggered aggregation results in AuNP’s peak absorbance quenching as well as red shift in the wavelength range of 520 to 543 nm. The colorimetric response at 520 nm is linear in the 2–100 ng mL−1 U concentration range, and the limit of detection is 0.3 ng mL−1. No interferences by other ions are found, and the relative standard deviation is ≤4% (for n = 5). The method is validated by analyzing a certified reference material (NIST SRM 1640a; natural water), and also applied to the quantification of U in four (spiked) water samples.

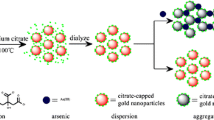
Schematic presentation of cloud point extraction (CPE) assisted coloirmetric and visual detection of uranium (U). In CPE of gold nanoparticles (AuNPs) the color of surfactant rich phase (SRP) turns red in absence of U(VI) and blue in presence of U(VI).






Similar content being viewed by others
References
Yang C-T, Han J, Gu M, Liu J, Li Y, Huang Z, Yu H-Z, Hu S, Wang X (2015) Fluorescent recognition of uranyl ions by a phosphorylated cyclic peptide. Chem Commun 51:11769–11772
Labrecque C, Potvin S, Whitty-Léveillé L, Larivièr D (2013) Cloud point extraction of uranium using H2DEH[MDP] in acidic conditions. Talanta 107:284–291
Saha A, Sanyal K, Rawat N, Deb SB, Saxena MK, Tomar BS (2017) Selective micellar extraction of Ultratrace levels of uranium in aqueous samples by task specific ionic liquid followed by its detection employing Total reflection X-ray fluorescence spectrometry. Anal Chem 89:10422–10430
Dutta RK, Kumar A (2016) Highly sensitive and selective method for detecting ultratrace levels of aqueous uranyl ions by strongly photoluminescent-responsive amine-modified cadmium sulfide quantum dots. Anal Chem 88:9071–9078
Rathore DPS (2008) Advances in technologies for the measurement of uranium in diverse matrices. Talanta 77:9–20
WHO (2011) Guidelines for drinking-water quality, 4th edn, Geneva
Common radionuclides found at superfund sites, EPA facts about uranium (2002) Environmental Protection Agency. In: US
AERB (2004) Drinking water specifications in India, Department of Atomic Energy, Govt. of India
Lorber A, Karpas Z, Halicz L (1996) Flow injection method for determination of uranium in urine and serum by inductively coupled plasma mass spectrometry. Anal Chim Acta 334:295–301
Misra NL, Dhara S, Singh Mudher KD (2006) Uranium determination in seawater by total reflection X-ray fluorescence spectrometry. Spectrochim Acta Part B 61:1166–1169
de Souza AL, Cotrim MEB, Pires MAF (2013) An overview of spectrometric techniques and sample preparation for the determination of impurities in uranium nuclear fuel grade. Microchim J 106:194–201
Brina R, Miller AG (1992) Direct detection of trace levels of uranium by laser-induced kinetic phosphorimetry. Anal Chem 64:1413–1418
Gwak R, Kim H, Yoo SM, Lee SY, Lee G-J, Lee M-K, Rhee C-K, Kang T, Kim B (2016) Precisely determining ultralow level UO2(2+) in natural water with Plasmonic nanowire interstice sensor. Sci Rep 6:19646
Amendola V, Pilot R, Frasconi M, Maragò OM, Iatì MA (2017) Surface plasmon resonance in gold nanoparticles: a review. J Phys Condens Matter 29:203002
Pezzato C, Maiti S, Chen JL-Y, Cazzolaro A, Gobbo C, Prins LJ (2015) Monolayer protected gold nanoparticles with metal-ion binding sites: functional systems for chemosensing applications. Chem Commun 51:9922–9931
Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA (2010) Gold nanoparticles for biology and medicine. Angew Chem Int Ed 49:3280–3294
Zhang D, Chen Z, Omar H, Deng L, Khashab NM (2015) Colorimetric peroxidase mimetic assay for uranyl detection in sea water. ACS Appl Mater Interfaces 7:4589–4594
Zhang H, Lin L, Zeng X, Ruan Y, Wu Y, Lin M, He Y, Fu F (2016) Magnetic beads-based DNAzyme recognition and AuNPs-based enzymatic catalysis amplification for visual detection of trace uranyl ion in aqueous environment. Biosens Bioelectron 78:73–79
Saha A, Debnath T, Neogy S, Ghosh HN, Saxena MK, Tomar BS (2017) Micellar extraction assisted fluorometric determination of ultratrace amount of uranium in aqueous samples by novel diglycolamide-capped quantum dot nanosensor. Sensors Actuators B 253:592–602
Ojeda CB, Rojas FS (2012) Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchim Acta 177:1–21
Saha A, Deb SB, Sarkar A, Saxena MK, Tomar BS (2016) Simultaneous preconcentration of uranium and thorium in aqueous samples using cloud point extraction. RSC Adv 6:20109–20119
Nair PR, Xavier M, Aggarwal SK (2009) A robost biamperometric titration methodology for the determination of uranium by Ti(III) reduction in presence of plutonium. Radiochim Acta 97:419–422
Castillo-López DN, Pal U (2014) Green synthesis of au nanoparticles using potato extract: stability and growth mechanism. J Nanopart Res 16:2571
Wagner CD, Riggs WM, Davis LE, Moulder JF, Muilenberg GE (1979) Handbook of X-ray photoelectron spectroscopy – a reference book of standard data for use in X-ray photoelectron spectroscopy. Perkin-Elmer Corporation, USA
Liu X, Atwater M, Wang J, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B 58:3–7
Zhang A, Asakura T, Uchiyama G (2003) The adsorption mechanism of uranium (VI) from seawater on a macroporous fibrous polymeric adsorbent containing amidoxime chelating functional group. React Funct Polym 57:67–76
Kim J, Tsouris C, Oyola Y, Janke CJ, Mayes RT, Dai S, Gill G, Kuo L-J, Wood J, Choe K-Y, Schneider E, Lindner H (2014) Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment. Ind Eng Chem Res 53:6076–6083
Kavakli PA, Seko N, Tamada M, Güven O (2004) A highly efficient chelating polymer for the adsorption of uranyl and vanadyl ions at low concentrations. Adsorption 10:309–315
Mehio N, Lashely MA, Nugent JW, Tucker L, Correia B, Do-Thanh C-L, Dai S, Hancock RD, Bryantsev VS (2015) Acidity of the amidoxime functional group in aqueous solution: a combined experimental and computational study. J Phys Chem B 119:3567–3576
Ugur I, Marion A, Parant S, Jensen JH, Monard G (2014) Rationalization of the pKa values of alcohols and thiols using atomic charge descriptors and its application to the prediction of amino acid pKa’s. J Chem Inf Model 54:2200–2213
J-f L, Liu R, Y-g Y, G-b J (2009) Triton X-114 based cloud point extraction: a thermoreversible approach for separation/concentration and dispersion of nanomaterials in the aqueous phase. Chem Commun:1514–1516
Tan Z-Q, Liu J-F, Liu R, Yin Y-G, Jiang G-B (2009) Visual and colorometric detection of Hg2+ by cloud point extraction with functionalized gold nanoparticles as a probe. Chem Commun:7030–7032
Schweitzer GK, Pesterfield LL (2010) The aqueous chemistry of the elements. Oxford University Press, New York
Orabi AH (2013) Determination of uranium after using solvent extraction from slightly nitric acid solution and spectrophotometric detection. J Radiat Res Appl Sci 6:1–10
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10:13–19
Acknowledgments
The authors are indebted to Dr. B.G. Vats, FCD, BARC and Dr. R. Chowdhury, BOD, BARC for their constant help throughout the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 3821 kb)
Rights and permissions
About this article
Cite this article
Saha, A., Neogy, S., Rao, D.R.M. et al. Colorimetric and visual determination of ultratrace uranium concentrations based on the aggregation of amidoxime functionalized gold nanoparticles. Microchim Acta 186, 183 (2019). https://doi.org/10.1007/s00604-019-3292-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3292-1