Abstract
Two dimensional single-crystal hexagonal gold nanosheets (SCHGNSs) were prepared by microwave heating of a solution of HAuCl4 in an ionic liquid. The SCHGNSs were characterized by field emission electron microscopy, energy-dispersive X-ray spectroscopy, X-ray diffraction, atomic force microscopy and electrochemical impedance spectroscopy. The SCHGNSs were then used to modify a graphite paste electrode for voltammetric determination of the hypertension drug captopril (CAP). The modified electrode showed a well-defined oxidation peak (at 0.41 V vs. Ag/AgCl) at pH 7.0 using differential pulse voltammetry. Under the optimum conditions, the response is linear in the 2–400 nM and 4.0–50 μM CAP concentration range, and the detection limit (at S/N = 3) is 0.3 nM. The sensor was successfully applied to the determination of CAP in pharmaceutical tablets and in spiked urine.

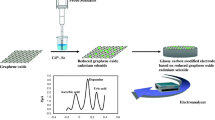
Schematic presentation of the preparation of single crystal hexagonal gold nanosheets and their use to modify a carbon paste electrode for ultra-trace voltammetric determination of the drug captopril







Similar content being viewed by others
References
Bouvy C, Baker GA, Yin H, Dai S (2010) Growth of gold nanosheets and nanopolyhedra in pyrrolidinium-based ionic liquids: investigation of the cation effect on the resulting morphologies. Cryst Growth Des 10(3):1319–1322
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34(4):257–264
Busbee BD, Obare SO, Murphy CJ (2003) An improved synthesis of high-aspect-ratio gold nanorods. Adv Mater 15(5):414–416
Huang X, Neretina S, El-Sayed MA (2009) Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv Mater 21(48):4880–4910
Hsu M-S, Chen Y-L, Lee C-Y, Chiu H-T (2012) Gold nanostructures on flexible substrates as electrochemical dopamine sensors. ACS Appl Mater Interfaces 4(10):5570–5575
Wang Y, Shan X, Wang H, Wang S, Tao N (2017) Plasmonic imaging of surface electrochemical reactions of single gold nanowires. J Am Chem Soc 139(4):1376–1379
Senger R, Dag S, Ciraci S (2004) Chiral single-wall gold nanotubes. Phys Rev Lett 93(19):196807
Wang L, Zhu Y, Wang J-Q, Liu F, Huang J, Meng X, Basset J-M, Han Y, Xiao F-S (2015) Two-dimensional gold nanostructures with high activity for selective oxidation of carbon–hydrogen bonds. Nat Commun 6:6957
Soejima T, Kimizuka N (2005) Ultrathin gold nanosheets formed by photoreduction at the ionic liquid/water interface. Chem Lett 34(9):1234–1235
Andoy NM, Zhou X, Choudhary E, Shen H, Liu G, Chen P (2013) Single-molecule catalysis mapping quantifies site-specific activity and uncovers radial activity gradient on single 2D nanocrystals. J Am Chem Soc 135(5):1845–1852
Tsuji M, Hashimoto M, Nishizawa Y, Tsuji T (2003) Preparation of gold nanoplates by a microwave-polyol method. Chem Lett 32(12):1114–1115
Li Z, Liu Z, Zhang J, Han B, Jimin D, Gao Y, Jiang T (2005) Synthesis of single-crystal gold nanosheets of large size in ionic liquids. J Phys Chem B 109(30):14445–14448
Lee JK, Kim D-C, Eui Song C, Lee S-g (2003) Thermal behaviors of ionic liquids under microwave irradiation and their application on microwave-assisted catalytic Beckmann rearrangement of ketoximes. Synth Commun 33(13):2301–2307
Berthold H, Schotten T, Honig H (2002) Transfer hydrogenation in ionic liquids under microwave irradiation. Synthesis 11:1607–1610
Dupont J (2004) On the solid, liquid and solution structural organization of imidazolium ionic liquids. J Braz Chem Soc 15(3):341–350
Shahrokhian S, Karimi M, Khajehsharifi H (2005) Carbon-paste electrode modified with cobalt-5-nitrolsalophen as a sensitive voltammetric sensor for detection of captopril. Sensors Actuators B Chem 109(2):278–284
Goodman A, Goodman L, Rall T, Murad F (1959) Las Bases Farmacologicas de la Terapeutica Panamericana. Spain, Madrid, pp 616–620
Karimi-Maleh H, Ensafi A, Allafchian A (2010) Fast and sensitive determination of captopril by voltammetric method using ferrocenedicarboxylic acid modified carbon paste electrode. J Solid State Electrochem 14(1):9
Heel R, Brogden R, Speight T, Avery G (1980) Captopril: a preliminary review of its pharmacological properties and therapeutic efficacy. Drugs 20(6):409–452
Huang T, He Z, Yang B, Shao L, Zheng X, Duan G (2006) Simultaneous determination of captopril and hydrochlorothiazide in human plasma by reverse-phase HPLC from linear gradient elution. J Pharm Biomed Anal 41(2):644–648
Rezende KR, Mundim IM, Teixeira LS, Souza WC, Ramos DR, Cardoso CR, Souza IC, Gratão MZ, Bellório KB (2007) Determination of captopril in human plasma, using solid phase extraction and high-performance liquid chromatography, coupled to mass spectrometry: application to bioequivalence study. J Chromatogr B 850(1–2):59–67
El-Shabrawy Y, El-Enany N, Salem K (2004) Sensitive kinetic spectrophotometric determination of captopril and ethamsylate in pharmaceutical preparations and biological fluids. Il Farmaco 59(10):803–808
Duncan FM, Martin VI, Williams BC (1983) Development and optimisation of a radioimmunoassay for plasma captopril. Clin Chim Acta 131(3):295–303
Absalan G, Akhond M, Karimi R, Ramezani AM (2018) Simultaneous determination of captopril and hydrochlorothiazide by using a carbon ionic liquid electrode modified with copper hydroxide nanoparticles. Microchim Acta 185(2):97
Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA (2010) Gold nanoparticles for biology and medicine. Angew Chem Int Ed 49(19):3280–3294
Frederix F, Bonroy K, Laureyn W, Reekmans G, Campitelli A, Dehaen W, Maes G (2003) Enhanced performance of an affinity biosensor interface based on mixed self-assembled monolayers of thiols on gold. Langmuir 19(10):4351–4357
Bard AJ, Faulkner LR, Leddy J, Zoski CG (1980) Electrochemical methods: fundamentals and applications, vol 2. wiley, New York
Zhang Q-L, Feng J-X, Wang A-J, Wei J, Lv Z-Y, Feng J-J (2015) A glassy carbon electrode modified with porous gold nanosheets for simultaneous determination of dopamine and acetaminophen. Microchim Acta 182(3–4):589–595
Moon GD, Lim GH, Song JH, Shin M, Yu T, Lim B, Jeong U (2013) Highly stretchable patterned gold electrodes made of au nanosheets. Adv Mater 25(19):2707–2712
Shetti NP, Malode SJ, Nandibewoor ST (2015) Electro-oxidation of captopril at a gold electrode and its determination in pharmaceuticals and human fluids. Anal Methods 7(20):8673–8682
Ribeiro PS, Santini A, Pezza HR, Pezza L (2003) Potentiometric determination of captopril in pharmaceutical formulations. Eclética Química 28(1):39–44
Soomro RA, Tunesi MM, Karakus S, Kalwar N (2017) Highly sensitive electrochemical determination of captopril using CuO modified ITO electrode: the effect of in situ grown nanostructures over signal sensitivity. RSC Adv 7(31):19353–19362
Niazi A, Pourghobadi Z, Nematollahi D, Beiginejad H (2014) Electrochemical oxidation and Voltammetric determination of captopril using 4, 4′-Biphenol as a homogeneous mediator. J Electrochem Soc 161(5):H284–H289
Raoof JB, Ojani R, Baghayeri M (2011) Sensitive voltammetric determination of captopril using a carbon paste electrode modified with nano-TiO2/ferrocene carboxylic acid. Chin J Catal 32(11–12):1685–1692
Rahman N, Anwar N, Kashif M, Hoda N (2006) A sensitive kinetic spectrophotometric method for the determination of captopril in bulk and dosage forms. Acta Pharma 56(3):347–357
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 161 kb)
Rights and permissions
About this article
Cite this article
Shahbakhsh, M., Noroozifar, M. 2D-Single-crystal hexagonal gold nanosheets for ultra-trace voltammetric determination of captopril. Microchim Acta 186, 195 (2019). https://doi.org/10.1007/s00604-019-3260-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3260-9