Abstract
Thin film nanofibers containing ZnTiO3 nanoparticles were used for rapid and efficient evaporation of solvents as used to extract analytes by dispersive liquid-liquid microextraction (DLLME). The method is referred to as thin film evaporation (TFE). A combination of DLLME and TFE was applied to the extraction and preconcentration of the pesticide chlorpyrifos (as a model compound) prior to analysis by ion mobility spectrometry. The ZnTiO3 nanoparticles were placed on polyacrylonitrile nanofibers which increases the porosity and surface area in the TFE process, thus causing fast and complete evaporation of solvents. The effects of sample pH value, extraction solvent and its volume, disperser solvent and volume, centrifugation time, evaporation time, and desorption temperature were optimized. The relative standard deviations of intra- and inter-day analyses were found to be 3% and 7%, respectively. The method has a linear dynamic range that covers the 0.10–3.0 μg.L−1 chlorpyrifos concentration range, the limit of detection is 0.04 μg.L−1, and the enrichment factor is up to 5400 in case of spiked samples. Some spiked field samples were analyzed and the relative recoveries ranged between 99 and 111%. When applied along with ion mobility spectrometry, the interferences caused by solvent are found to be reduced in the ionization source. This makes it possible to select a variety of solvents as needed for sample extraction.

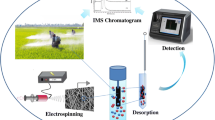
Schematic presentation of dispersive liquid-liquid microextraction (DLLME) combined with thin film evaporation (TFE) for rapid vaporization of extraction solvent: Application to the pre-concentration of chlorpyrifos prior to its quantification by corona discharge-ion mobility spectrometry (CD-IMS).





Similar content being viewed by others
References
Sorribes-Soriano A, de la Guardia M, Esteve-Turrillas FA, Armenta S (2018) Trace analysis by ion mobility spectrometry: from conventional to smart sample preconcentration methods. A review. Anal Chim Acta 1026:37–50. https://doi.org/10.1016/j.aca.2018.03.059
Jafari MT, Rezaei B, Zaker B (2009) Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal Chem 81:3585–3591. https://doi.org/10.1021/ac802557t
Cavanna D, Zanardi S, Dall'Asta C, Suman M (2019) Ion mobility spectrometry coupled to gas chromatography: a rapid tool to assess eggs freshness. Food Chem 271:691–696. https://doi.org/10.1016/j.foodchem.2018.07.204
Jafari MT, Khayamian T (2009) Simultaneous determination of 2-furfural and 5-methyl-2-furfural using corona discharge ion mobility spectrometry, Anal Sci 25:801–805. https://doi.org/10.2116/analsci.25.801
Jafari MT, Saraji M, Ameri AH (2015) Coupling of solid phase microextraction with electrospray ionization ion mobility spectrometry and direct analysis of venlafaxine in human urine and plasma. Anal Chim Acta 853:460–468. https://doi.org/10.1016/j.aca.2014.10.054
Hashemian Z, Khayamian T, Saraji M (2015) Anticodeine aptamer immobilized on a Whatman cellulose paper for thin-film microextraction of codeine from urine followed by electrospray ionization ion mobility spectrometry. Anal Bioanal Chem 407:1615–1623. https://doi.org/10.1007/s00216-014-8392-5
Jafari MT, Rezayat MR, Mossaddegh M (2018) Design and construction of an injection port for coupling stir-bar sorptive extraction with ion mobility spectrometry. Talanta 178:369–376. https://doi.org/10.1016/j.talanta.2017.09.061
Márquez-Sillero I, Aguilera-Herrador E, Cárdenas S, Valcárcel M (2011) Determination of 2,4,6-tricholoroanisole in water and wine samples by ionic liquid-based single-drop microextraction and ion mobility spectrometry. Anal Chim Acta 702:199–204. https://doi.org/10.1016/j.aca.2011.06.046
Ebrahimi A, Jafari MT (2015) Negative corona discharge-ion mobility spectrometry as a detection system for low density extraction solvent-based dispersive liquid–liquid microextraction. Talanta 134:724–731. https://doi.org/10.1016/j.talanta.2014.12.018
Bruheim I, Liu X, Pawliszyn J (2003) Thin-film microextraction. Anal Chem 75:1002–1010. https://doi.org/10.1021/ac026162q
Qin Z, Bragg L, Ouyang G, Pawliszyn J (2008) Comparison of thin-film microextraction and stir bar sorptive extraction for the analysis of polycyclic aromatic hydrocarbons in aqueous samples with controlled agitation conditions. J Chromatogr A 1196-1197:89–95. https://doi.org/10.1016/j.chroma.2008.03.063
Jiang R, Pawliszyn J (2012) Thin-film microextraction offers another geometry for solid phase microextraction. TrAC-Trends Anal Chem 39:245–253. https://doi.org/10.1016/j.trac.2012.07.005
Yamaguchi MS, McCartney MM, Linderholm AL, Ebeler SE, Schivo M, Davis CE (2018) Headspace sorptive extraction-gas chromatography–mass spectrometry method to measure volatile emissions from human airway cell cultures. J Chromatogr A 1090:36–42. https://doi.org/10.1016/j.jchromb.2018.05.009
Ilias Y, Bieri S, Christen P, Veuthey JL (2006) Evaluation of solid-phase microextraction desorption parameters for fast GC analysis of cocaine in coca leaves. J Chromatogr Sci 44:394–398. https://doi.org/10.1093/chromsci/44.7.394
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9. https://doi.org/10.1016/j.chroma.2006.03.007
Jafari MT, Riahi F (2014) Feasibility of corona discharge ion mobility spectrometry for direct analysis of samples extracted by dispersive liquid–liquid microextraction. J Chromatogr A 1343:63–68. https://doi.org/10.1016/j.chroma.2014.03.069
Farajzadeh MA, Feriduni B, Mogaddam MRA (2016) Development of a new extraction method based on counter current salting-out homogenous liquid–liquid extraction followed by dispersive liquid–liquid microextraction: application for the extraction and preconcentration of widely used pesticides from fruit juices. Talanta 146:772–779. https://doi.org/10.1016/j.talanta.2015.06.024
Jafari MT, Saraji M, Mossaddegh M (2016) Combination of dispersive liquid–liquid microextraction and solid–phase microextraction: an efficient hyphenated sample preparation method. J Chromatogr A 1466:50–58. https://doi.org/10.1016/j.chroma.2016.09.015
Chi Y, Yuan Q, Hou S, Zhao Z (2016) Synthesis and characterization of mesoporous ZnTiO3 rods via a polyvinylpyrrolidone assisted sol–gel method. Ceram Int 42:5094–5099. https://doi.org/10.1016/j.ceramint.2015.12.024
Saraji M, Jafari MT, Mossaddegh M (2016) Halloysite nanotubes–titanium dioxide as a solid phase microextraction coating combined with negative corona discharge–ion mobility spectrometry for the determination of parathion. Anal Chim Acta 926:55–62. https://doi.org/10.1016/j.aca.2016.04.034
Mallakpour S, Hatami M (2017) Biosafe organic diacid intercalated LDH/PVC nanocomposites versus pure LDH and organic diacid intercalated LDH: synthesis, characterization and removal behaviour of Cd2+ from aqueous test solution. Appl Clay Sci 149:28–40. https://doi.org/10.1016/j.clay.2017.09.006
Xiong J, Hu B (2008) Comparison of hollow fiber liquid phase microextraction and dispersive liquid–liquid microextraction for the determination of organosulfur pesticides in environmental and beverage samples by gas chromatography with flame photometric detection. J Chromatogr A 1193:7–18. https://doi.org/10.1016/j.chroma.2008.03.072
Ghavidel F, Shahtaheri SJ, Torabbeigi M, Froushani AR (2014) Microwave assisted head space solid phase microextraction for analysis of butachlor and chlorpyrifos pesticides in urine. Anal Chem Lett 4:224–231. https://doi.org/10.1080/22297928.2014.995704
Ghotbadini-Bahraman N, Sheibani A, Shishehbore MR (2017) Off-line coupling of QuEChERS sample preparation to ion mobility spectrometry for the determination of chlorpyrifos residue in pistachio oil. Int J Ion Mobil Spec 20:41–45. https://doi.org/10.1007/s12127-017-0214-y
Saraji M, Jafari MT, Sherafatmand H (2015) Sol–gel/nanoclay composite as a solid-phase microextraction fiber coating for the determination of organophosphorus pesticides in water samples. Anal Bioanal Chem 407:1241–1252. https://doi.org/10.1007/s00216-014-8344-0
Gabaldón JA, Maquieira A, Puchades R (2007) Development of a simple extraction procedure for chlorpyrifos determination in food samples by immunoassay. Talanta 71:1001–1010. https://doi.org/10.1016/j.talanta.2006.04.041
Sanagi MM, Salleh S, Ibrahim WAW, Naim AA, Hermawan D, Miskam M, Hussain I, Aboul-Enein HY (2013) Molecularly imprinted polymer solid-phase extraction for the analysis of organophosphorus pesticides in fruit samples. J Food Compos Anal 32:155–161. https://doi.org/10.1016/j.jfca.2013.09.001
Rousis NI, Zuccato E, Castiglioni S (2016) Monitoring population exposure to pesticides based on liquid chromatography-tandem mass spectrometry measurement of their urinary metabolites in urban wastewater: a novel biomonitoring approach. Sci Total Environ 571:1349–1357. https://doi.org/10.1016/j.scitotenv.2016.07.036
Mehrani Z, Ebrahimzadeh H, Aliakbar AR, Asgharinezhad AA (2018) A poly(4 nitroaniline)/poly(vinyl alcohol) electrospun nanofiber as an efficient nanosorbent for solid phase microextraction of diazinon and chlorpyrifos from water and juice samples. Microchim Acta 185:384. https://doi.org/10.1007/s00604-018-2911-6
Bagheri H, Amanzadeh H, Yamini Y, Masoomi MY, Morsali A, Salar-Amoli J, Hassan J (2018) A nanocomposite prepared from a zinc-based metal-organic framework and polyethersulfone as a novel coating for the headspace solid-phase microextraction of organophosphorous pesticides. Microchim Acta 185:62. https://doi.org/10.1007/s00604-017-2607-3
Li C, Chen L, Li W (2013) Magnetic titanium oxide nanoparticles for hemimicelle extraction and HPLC determination of organophosphorus pesticides in environmental water. Microchim Acta 180:1109–1116. https://doi.org/10.1007/s00604-013-1029-0
Acknowledgments
The authors would like to thank the Research Council of Isfahan University of Technology (IUT) and Center of Excellence in Sensor and Green Chemistry, Iran for providing the financial support of this work. Dr. Bahrami is also specially acknowledged for her valuable assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
Rezayat, M.R., Jafari, M.T. & Rahmanian, F. Thin film nanofibers containing ZnTiO3 nanoparticles for rapid evaporation of extraction solvent: application to the preconcentration of chlorpyrifos prior to its quantification by ion mobility spectrometry. Microchim Acta 186, 35 (2019). https://doi.org/10.1007/s00604-018-3167-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3167-x