Abstract
A method is described for the chemiluminescence based determination of the activity of catalase (CAT) using H2O2-sensitive CdTe quantum dots (QDs). It is based on the finding that the chemiluminescence (CL) of the CdTe/H2O2 system is reduced due to the consumption of H2O2 by the catalytic action of CAT. The Michaelis constant is calculated to be 519 ± 27 mM, showing the potential of the method to accurately measure the Km compared to the standard method. The method does not require QDs to be conjugated to biological/organic molecules and therefore is considered to be a rapid and convenient method for determination of CAT in real samples. At an incubation time of 2 s, the LOD was calculated to be 4.5 unit/mL, with a linear range from 6 to 400 unit/mL. The assay is sensitive, simple, and suitable for practical applications.

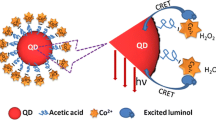
Schematic representation of chemiluminescence-based catalase U(CAT) assay using the CdSe QD/H2O2 system. The reduction of H2O2 is reflected by the chemiluminescence of the QDs. A mechanism is put forward based on the changes in chemiluminescence intensity of the QDs by the consumption of H2O2 due to the catalytic action of CAT.






Similar content being viewed by others
References
Yang Y, Chen O, Angerhofer A, Cao YC (2006) Radial-position-controlled doping in CdS/ZnS core/shell nanocrystals. J Am Chem Soc 128:12428–12429
Huang C-P, Li Y-K, Chen T-M (2007) A highly sensitive system for urea detection by using CdSe/ZnS core-shell quantum dots. Biosens Bioelectron 22:1835–1838
Powe AM, Fletcher KA, St. Luce NN et al (2004) Molecular fluorescence, phosphorescence, and chemiluminescence spectrometry. Anal Chem 76:4614–4634
King DW, Cooper WJ, Rusak SA et al (2007) Flow injection analysis of H2O2 in natural waters using acridinium ester chemiluminescence: method development and optimization using a kinetic model. Anal Chem 79:4169–4176
Wang Z, Li J, Liu B et al (2005) Chemiluminescence of CdTe nanocrystals induced by direct chemical oxidation and its size-dependent and surfactant-sensitized effect. J Phys Chem B 109:23304–23311
Frigerio C, Ribeiro DSM, Rodrigues SSM et al (2012) Application of quantum dots as analytical tools in automated chemical analysis: a review. Anal Chim Acta 735:9–22
Chen H, Lin L, Li H, Lin J-M (2014) Quantum dots-enhanced chemiluminescence: mechanism and application. Coord Chem Rev 263:86–100
Chen H, Xue W, Lu C et al (2013) Plasmonic luminescent core–shell nanocomposites-enhanced chemiluminescence arising from the decomposition of peroxomonosulfite. Spectrochim Acta A Mol Biomol Spectrosc 116:355–360
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61:192–208
Abdel-Mageed HM, El-Laithy HM, Mahran LG et al (2012) Development of novel flexible sugar ester vesicles as carrier systems for the antioxidant enzyme catalase for wound healing applications. Process Biochem 47:1155–1162
Xu F (2005) Applications of oxidoreductases: recent progress. Ind Biotechnol 1:38–50
Ogura Y (1955) Catalase activity at high concentration of hydrogen peroxide. Arch Biochem Biophys 57:288–300
George P (1947) Reaction between catalase and hydrogen peroxide. Nature 159:41–43
George P (1949) The effect of the peroxide concentration and other factors on the decomposition of hydrogen peroxide by catalase. Biochem J 44:197
Bonnichsen RK, Chance B, Theorell H (1947) Catalase activity. Acta Chem Scand 1:685–709
Ozyilmaz G, Tukel SS, Alptekin O (2007) Kinetic properties and storage stability of catalase immobilized on to florisil
Vetrano AM, Heck DE, Mariano TM et al (2005) Characterization of the oxidase activity in mammalian catalase. J Biol Chem 280:35372–35381
Mozaffar S, Ueda M, Kitatsuji K et al (1986) Properties of catalase purified from a methanol-grown yeast, Kloeckera sp. 2201. FEBS J 155:527–531
Alptekin Ö, Tükel SS, Yıldırım D, Alagöz D (2010) Immobilization of catalase onto Eupergit C and its characterization. J Mol Catal B Enzym 64:177–183
Alptekin Ö, Tükel SS, Yildirim D (2008) Immobilization and characterization of bovine liver catalase on eggshell. J Serb Chem Soc 73:609–618
Çetinus ŞA, Öztop HN (2000) Immobilization of catalase on chitosan film. Enzym Microb Technol 26:497–501
Vasudevan PT, Weiland RH (1990) Deactivation of catalase by hydrogen peroxide. Biotechnol Bioeng 36:783–789
Chance B, Greenstein DS, Roughton FJW (1952) The mechanism of catalase action. I. Steady-state analysis. Arch Biochem Biophys 37:301–321
Haber J, Maśalakiewicz P, Rodakiewicz-nowak J, Walde P (1993) Activity and spectroscopic properties of bovine liver catalase in sodium bis (2-ethylhexyl) sulfosuccinate/isooctane reverse micelles. FEBS J 217:567–573
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Gharaat M, Sajedi RH, Shanehsaz M et al (2017) A dextran mediated multicolor immunochromatographic rapid test strip for visual and instrumental simultaneous detection of vibrio cholera O1 (Ogawa) and clostridium botulinum toxin a. Microchim Acta 184:4817–4825
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Zhao Y, Zhao S, Huang J, Ye F (2011) Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 85:2650–2654
Periasamy AP, Umasankar Y, Chen S-M (2009) Nanomaterials-acetylcholinesterase enzyme matrices for organophosphorus pesticides electrochemical sensors: a review. Sensors 9:4034–4055
Rongen HAH, Hoetelmans RMW, Bult A, Van Bennekom WP (1994) Chemiluminescence and immunoassays. J Pharm Biomed Anal 12:433–462
Liang A, Liang Y, Jiang Z, Jiang H (2009) Resonance scattering spectral detection of catalase activity using au@ ag nanoparticle as probe and coupling catalase catalytic reaction with Fenton reaction. J Fluoresc 19:1009
Wu M, Lin Z, Wolfbeis OS (2003) Determination of the activity of catalase using a europium (III)–tetracycline-derived fluorescent substrate. Anal Biochem 320:129–135
Deng H-H, Wu G-W, He D et al (2015) Fenton reaction-mediated fluorescence quenching of N-acetyl-L-cysteine-protected gold nanoclusters: analytical applications of hydrogen peroxide, glucose, and catalase detection. Analyst 140:7650–7656
Escobar L, Salvador C, Contreras M, Escamilla JE (1990) On the application of the Clark oxygen electrode to the study of enzyme kinetics in apolar solvents: the catalase reaction. Anal Biochem 184:139–144
Fiedurek J, Gromada A (2000) Production of catalase and glucose oxidase by aspergillus Niger using unconventional oxygenation osf culture. J Appl Microbiol 89:85–89
Acknowledgments
The authors express their gratitude to the research council of Tarbiat Modares University and Ministry of Sciences, Researches, and Technology for financial support during the course of this project. We also thank Hossein Rahmani for his guidance with this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 424 kb)
Rights and permissions
About this article
Cite this article
Ghavamipour, F., Sajedi, R.H. & Khajeh, K. A chemiluminescence-based catalase assay using H2O2-sensitive CdTe quantum dots. Microchim Acta 185, 376 (2018). https://doi.org/10.1007/s00604-018-2912-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2912-5