Abstract
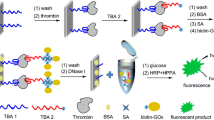
The article describes an on-chip amplification scheme initiated by a terminal deoxynucleotidyl transferase (TdT) for highly sensitive fluorometric determination of protein. Two thrombin-binding aptamers were designed to capture thrombin as they can form a sandwich structure for improved specificity. An amino-modified aptamer (TBA29) was first immobilized on a silicon chip. After capture of thrombin, a second aptamer (TBA15) was conjugated to the second binding site of thrombin. The 3’-terminal of aptamer TBA15 is exposed on the chip surface, and then fluorescein-labeled 12-dATP associates to the 3’-terminal with the help of TdT. This results in signal amplification, and eventually leads to highly sensitive detection. Under optimal conditions, fluorescence intensity is linearly related to the logarithm of thrombin concentration in the range of 100 fM - 0.1 μM, and the detection limit is as low as 2.0 fM. The assay is sensitive and selective even over potentially interfering proteins and in the presence of human serum.

Schematic strategy for thrombin detection. Two thrombin-binding aptamers were designed to capture thrombin to form a sandwich structure for improved specificity. The protein detection is based on TdT initiated on-chip fluorescent amplification.





Similar content being viewed by others
References
Cao Y, Chen D, Chen W, Yu J, Chen Z, Li G (2014) Aptamer-based homogeneous protein detection using cucurbit [7] uril functionalized electrode. Anal Chim Acta 812:45–49 https://doi.org/10.1016/j.aca.2014.01.008
Cao Y, Wang Z, Cao J, Mao X, Chen G, Zhao J (2017) A general protein aptasensing strategy based on untemplated nucleic acid elongation and the use of fluorescent copper nanoparticles: Application to the detection of thrombin and the vascular endothelial growth factor. Microchim Acta 184:3697–3704 https://doi.org/10.1007/s00604-017-2393-y
Shi K, Dou B, Yang J, Yuan R, Xiang Y (2017) Target-triggered catalytic hairpin assembly and TdT-catalyzed DNA polymerization for amplified electronic detection of thrombin in human serums. Biosens Bioelectron 87:495–500 https://doi.org/10.1016/j.bios.2016.08.056
Zhang L, Li L (2016) Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim Acta 183:485–490 https://doi.org/10.1007/s00604-015-1674-6
Kumar Mishra S, Kumar A (2016) NALDB: nucleic acid ligand database for small molecules targeting nucleic acid. Database:baw002 https://doi.org/10.1093/database/baw002
Tanaka T, Matsunaga T (2000) Fully automated chemiluminescence immunoassay of insulin using antibody- protein A- bacterial magnetic particle complexes. Anal Chem 72:3518–3522 https://doi.org/10.1021/ac9912505
Wang K, Liao J, Yang X, Zhao M, Chen M, Yao W, Lan X (2015) A label-free aptasensor for highly sensitive detection of ATP and thrombin based on metal-enhanced PicoGreen fluorescence. Biosens Bioelectron 63:172–177 https://doi.org/10.1016/j.bios.2014.07.022
Tjong V (2013) On-chip labeling via surface initiated enzymatic polymerization (SIEP) for nucleic acids hybridization detection. Dissertation, Duke University http://hdl.handle.net/10161/7152. Accessed 13 May 2013
Zhang X, Li S, Jin X, Zhang S (2011) A new photoelectrochemical aptasensor for the detection of thrombin based on functionalized graphene and CdSe nanoparticles multilayers. Chem Commun 47:4929–4931 https://doi.org/10.1039/C1CC10830A
Sui N, Wang L, Xie F, Liu F, Xiao H, Liu M, William WY (2016) Ultrasensitive aptamer-based thrombin assay based on metal enhanced fluorescence resonance energy transfer. Microchim Acta 183:1563–1570. https://doi.org/. https://doi.org/10.1007/s00604-016-1774-y
Xiang Y, Xie M, Bash R, Chen JJ, Wang J (2007) Ultrasensitive Label-Free Aptamer-Based Electronic Detection. Angew Chem Int Ed 46:9054 https://doi.org/10.1002/anie.200703242
Barreda-García S, González-Álvarez MJ, de-los Santos-Álvarez N, Palacios-Gutiérrez JJ, Miranda-Ordieres AJ, Lobo-Castañón MJ (2015) Attomolar quantitation of Mycobacterium tuberculosis by asymmetric helicase-dependent isothermal DNA-amplification and electrochemical detection. Biosens Bioelectron 68:122–128 https://doi.org/10.1016/j.bios.2014.12.029
Miao M, Tian J, Luo Y, Du Z, Liang Z, Xu W (2018) Terminal deoxynucleotidyl transferase-induced DNAzyme nanowire sensor for colorimetric detection of lipopolysaccharides. Sensors Actuators B Chem 256:790–796 https://doi.org/10.1016/j.snb.2017.10.004
Xie S, Chai Y, Yuan Y, Bai L, Yuan R (2014) A novel electrochemical aptasensor for highly sensitive detection of thrombin based on the autonomous assembly of hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme nanowires. Anal Chim Acta 832:51–57 https://doi.org/10.1016/j.aca.2014.04.065
Tang Z, Zhang H, Ma C, Gu P, Zhang G, Wu K, Wang K (2018) Colorimetric determination of the activity of alkaline phosphatase based on the use of Cu (II)-modulated G-quadruplex-based DNAzymes. Mikrochim Acta 185(109) https://doi.org/10.1007/s00604-017-2628-y
Meirinho SG, Dias LG, Peres AM, Rodrigues LR (2017) Rodrigues, Electrochemical aptasensor for human osteopontin detection using a DNA aptamer selected by SELEX. Anal Chim Acta 987:25–37 https://doi.org/10.1016/j.aca.2017.07.071
Chow DC, Chilkoti A (2007) Surface-initiated enzymatic polymerization of DNA. Langmuir 23:11712–11717 https://doi.org/10.1021/la701630g
Jung J, Hyun J (2011) Visualization of enzymatic DNA extension by surface plasmon resonance imaging. Biochip J 5:304–308 https://doi.org/10.1007/s13206-011-5403-x
Motea EA, Berdis AJ (2010) Terminal deoxynucleotidyl transferase: the story of a misguided DNA polymerase. Biochim Biophys Acta 1804:1151–1166 https://doi.org/10.1016/j.bbapap.2009.06.030
Kool E T (2006) Use of Multiple Fluorescent Labels in Biological Sensing, Stanford Univ Ca Dept Of Chemistry.
Zhao H, Liu Q, Liu M, Jin Y, Li B (2017) Label-free fluorescent assay of T4 polynucleotide kinase phosphatase activity based on G-quadruplexe− thioflavin T complex. Talanta 165:653–658 https://doi.org/10.1016/j.talanta.2017.01.027
Cho Y, Kool ET (2006) Enzymatic synthesis of fluorescent oligomers assembled on a DNA backbone. Chembiochem 7:669–672 https://doi.org/10.1002/cbic.200500515
Tjong V, Yu H, Hucknall A, Chilkoti A (2012) Direct fluorescence detection of RNA on microarrays by surface-initiated enzymatic polymerization. Anal Chem 85:426–433 https://doi.org/10.1021/ac303132j
Wan Y, Xu H, Su Y, Zhu X, Song S, Fan C (2013) A surface-initiated enzymatic polymerization strategy for electrochemical DNA sensors. Biosens Bioelectron 41:526–531 https://doi.org/10.1016/j.bios.2012.09.017
Lenigk R, Carles M, Ip NY, Sucher NJ (2001) Surface characterization of a silicon-chip-based DNA microarray. Langmuir 17:2497–2501 https://doi.org/10.1021/la001355z
Hu W, Hu Q, Li L, Kong J, Zhang X (2015) Detection of sequence-specific DNA with a morpholino-functionalized silicon chip. Anal Methods 7:2406–2412 https://doi.org/10.1039/C4AY02780A
Tasset DM, Kubik MF, Steiner W (1997) Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J Mol Biol 272:688–698 https://doi.org/10.1006/jmbi.1997.1275
Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ (1992) Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 355:564–566 https://doi.org/10.1038/355564a0
Lao YH, Peck K, Chen LC (2009) Enhancement of aptamer microarray sensitivity through spacer optimization and avidity effect. Anal Chem 81:1747–1754 https://doi.org/10.1021/ac801285a
Kim Y, Cao Z, Tan W (2008) Molecular assembly for high-performance bivalent nucleic acid inhibitor. Proc Natl Acad Sci U S A 105:5664–5669 https://doi.org/10.1073/pnas.0711803105
Zhang XF, Zhang J, Liu L (2014) Fluorescence properties of twenty fluorescein derivatives: lifetime, quantum yield, absorption and emission spectra. J Fluoresc 24:819–826 https://doi.org/10.1007/s10895-014-1356-5
Sjöback R, Nygren J, Kubista M (1995) Absorption and fluorescence properties of fluorescein. Spectrochim Acta A Mol Biomol Spectrosc 51:7–21 https://doi.org/10.1016/0584-8539(95)01421-P
Bai S, Wang T, Zhang Z, Sheng S, Yu W, Xie G (2017) A novel colorimetric biosensor for detecting target DNA and human alpha thrombin based on associative toehold activation concatemer induced catalyzed hairpin assembly amplification. Sensors Actuators B Chem 239:447–454 https://doi.org/10.1016/j.snb.2016.08.026
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 21575066) and Henan University of Chinese Medicine of graduate student innovation training base project (grant No 2017YCX037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 469 kb)
Rights and permissions
About this article
Cite this article
Wen, D., He, M., Ma, K. et al. Highly sensitive fluorometric determination of thrombin by on-chip signal amplification initiated by terminal deoxynucleotidyl transferase. Microchim Acta 185, 380 (2018). https://doi.org/10.1007/s00604-018-2903-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2903-6