Abstract
The authors present a voltammetric immunoassay for the quantification of the prostate specific antigen (PSA), a well-established tumor marker. Biotinylated anti- PSA antibodies were immobilized on the surface of a nano-TiO2-modified carbon paste electrode using specific interaction between streptavidin and biotin. Streptavidin itself was covalently grafted to the electrode surface via 4-aminobenzoic acid film. Signal transduction was then performed using polyclonal antibodies conjugated with horseradish peroxidase (HRP) and thionine. An amplified catalytic reduction current was observed in the presence of H2O2 because more than one polyclonal antibody linked to each antigen. Each step of the preparation of the immunosensor was monitored via electrochemical impedance spectroscopy. The electrode, if operated at a typical potential of 0.3 V vs. Ag/AgCl and using hexacyanoferrate as an electrochemical probe, exhibits linear responses in the 0.10 to 5.0 ng·mL−1 and 5.0 to 100 ng·mL−1 PSA concentration ranges, with a detection limit of 200 pg·mL−1. The accuracy of the biosensor was confirmed by analyzing a certified human serum sample, and this indicated that the immunosensor is well suited for the quantification of PSA.

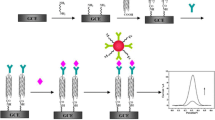
Schematic presentation of a sandwich-type electrochemical immunosensor for the prostate specific antigen. It is based on the use of a carbon paste electrode, streptavidin and biotinylated monoclonal antibody. Signal transduction is the result of the catalytic reduction of thionine.




Similar content being viewed by others
References
Srinivas PR, Kramer BS, Srivastava S (2001) Trends in biomarker research for cancer detection. Lancet Oncol 2(11):698–704
Tothill IE (2009) Biosensors for cancer markers diagnosis. Semin Cell Dev Biol 20(1):55–62
Faraggi D, Kramar A (2000) Methodological issues associated with tumor marker development: biostatistical aspects. In: Urologic oncology: seminars and original investigations. Elsevier, Amsterdam
Szilvás Á, Blázovics A, Székely G, Dinya E, Fehér J, Mózsik G (2001) Comparative study between the free radicals and tumor markers in patients with gastrointestinal tumors. J Physiol Paris 95(1):247–252
Liu Z-H, Zhang G-F, Chen Z, Qiu B, Tang D (2014) Prussian blue-doped nanogold microspheres for enzyme-free electrocatalytic immunoassay of p53 protein. Microchim Acta 181(5–6):581–588
Xiong J, Wang W, Zhou Y, Kong W, Wang Z, Fu Z (2016) Ultra-sensitive chemiluminescent detection of Staphylococcus aureus based on competitive binding of staphylococcus protein A-modified magnetic beads to immunoglobulin G. Microchim Acta 183(4):1507–1512
Röwer C, Vissers JP, Koy C, Kipping M, Hecker M, Reimer T, Gerber B, Thiesen H-J, Glocker MO (2009) Towards a proteome signature for invasive ductal breast carcinoma derived from label-free nanoscale LC-MS protein expression profiling of tumorous and glandular tissue. Anal Bioanal Chem 395(8):2443–2456
Lee S, Hosokawa K, Kim S, Jeong OC, Lilja H, Laurell T, Maeda M (2016) Porous silicon microarray for simultaneous fluorometric immunoassay of the biomarkers prostate-specific antigen and human glandular kallikrein 2. Microchim Acta 183(12):3321–3327
Miao L, Jiao L, Zhang J, Li H (2017) Amperometric sandwich immunoassay for the carcinoembryonic antigen using a glassy carbon electrode modified with iridium nanoparticles, polydopamine and reduced graphene oxide. Microchim Acta 184(1):169–175
Krivitsky V, Zverzhinetsky M, Patolsky F (2016) Antigen-dissociation from antibody-modified Nanotransistor sensor arrays as a direct biomarker detection method in unprocessed Biosamples. Nano Lett 16(10):6272–6281
Choi JH, Kim HS, Choi J-W, Hong JW, Kim Y-K, Oh B-K (2013) A novel au-nanoparticle biosensor for the rapid and simple detection of PSA using a sequence-specific peptide cleavage reaction. Biosens Bioelectron 49:415–419
Souada M, Piro B, Reisberg S, Anquetin G, Noël V, Pham M (2015) Label-free electrochemical detection of prostate-specific antigen based on nucleic acid aptamer. Biosens Bioelectron 68:49–54
Su L-C, Chen R-C, Li Y-C, Chang Y-F, Lee Y-J, Lee C-C, Chou C (2010) Detection of prostate-specific antigen with a paired surface plasma wave biosensor. Anal Chem 82(9):3714–3718
Knopp D (2006) Immunoassay development for environmental analysis. Anal Bioanal Chem 385(3):425–427
Zhao Y, Zheng Y, Kong R, Xia L, Qu F (2016) Ultrasensitive electrochemical immunosensor based on horseradish peroxidase (HRP)-loaded silica-poly(acrylic acid) brushes for protein biomarker detection. Biosens Bioelectron 75:383–388
Qu F, Sun H, Zhang S, You J (2012) Electrochemical sensing platform based on palladium modified ceria nanoparticles. Electrochim Acta 61:173–178
Qu F, Li T, Yang M (2011) Colorimetric platform for visual detection of cancer biomarker based on intrinsic peroxidase activity of graphene oxide. Biosens Bioelectron 26:3927–3931
Qu F, Lu H, Yang M, Deng C (2011) Electrochemical immunosensor based on electron transfer mediated by graphene oxide initiated silver enhancement. Biosens Bioelectron 26:4810–4814
Lin Y, Zhou Q, Lin Y, Tang D, Niessner R, Knopp D (2015) Enzymatic hydrolysate-induced displacement reaction with multifunctional silica beads doped with horseradish peroxidase–Thionine conjugate for ultrasensitive electrochemical immunoassay. Anal Chem 87(16):8531–8540
Pavasupree S, Jitputti J, Ngamsinlapasathian S, Yoshikawa S (2008) Hydrothermal synthesis, characterization, photocatalytic activity and dye-sensitized solar cell performance of mesoporous anatase TiO2 nanopowders. Mat Res Bull 43:149–157
Wild D (2005) The immunoassay handbook. Elsevier, Amsterdam
Xiao Y, Ju HX, Chen HY (1999) A reagentless hydrogen peroxide sensor based on incorporation of horseradish peroxidase in poly (thionine) film on a monolayer modified electrode. Anal Chim Acta 391:299–306
Ojeda I, López-Montero J, Moreno-Guzmán M, Janegitz B, González-Cortés A, Yáñez-Sedeño P, Pingarron J (2012) Electrochemical immunosensor for rapid and sensitive determination of estradiol. Anal Chim Acta 743:117–124
Liu J, Cheng L, Liu B, Dong S (2000) Covalent modification of a glassy carbon surface by 4-aminobenzoic acid and its application in fabrication of a polyoxometalates-consisting monolayer and multilayer films. Langmuir 16(19):7471–7476
H-w R, Y-s K, Lee J-h, W-s S, N-s M, Hong H-G (2006) Immobilization of horseradish peroxidase to electrochemically deposited gold-nanoparticles on glassy carbon electrode for determination of H2O2. Bull Kor Chem Soc 27(5):672–678
Paredes J, Villar-Rodil S, Martínez-Alonso A, Tascon J (2008) Graphene oxide dispersions in organic solvents. Langmuir 24(19):10560–10564
Nassar A-EF, Willis WS, Rusling JF (1995) Electron transfer from electrodes to myoglobin: facilitated in surfactant films and blocked by adsorbed biomacromolecules. Anal Chem 67(14):2386–2392
Pei R, Cheng Z, Wang E, Yang X (2001) Amplification of antigen–antibody interactions based on biotin labeled protein–streptavidin network complex using impedance spectroscopy. Biosens Bioelectron 16(6):355–361
Zhao J, Guo Z, Feng D, Guo J, Wang J, Zhang Y (2015) Simultaneous electrochemical immunosensing of alpha-fetoprotein and prostate specific antigen using a glassy carbon electrode modified with gold nanoparticle-coated silica nanospheres and decorated with azure a or ferrocenecarboxylic acid. Microchim Acta 182(15):2435–2442
Sun XC, Lei C, Guo L, Zhou Y (2016) Sandwich immunoassay for the prostate specific antigen using a micro-fluxgate and magnetic bead labels. Microchim Chim Acta 183(8):2385–2393
Tahmasebi F, Noorbakhsh A (2016) Sensitive electrochemical prostate specific antigen Aptasensor: effect of carboxylic acid functionalized carbon nanotube and glutaraldehyde linker. Electroanalysis 28(5):1134–1145
Biscay J, González García MB, García AC (2015) Determination of Total PSA using magnetic beads and a re-usable screen printed carbon electrode Array. Electroanalysis 27(12):2773–2777
Wang Y, Qu Y, Liu G, Hou X, Huang Y, Wu W, Wu K, Li C (2015) Electrochemical immunoassay for the prostate specific antigen using a reduced graphene oxide functionalized with a high molecular-weight silk peptide. Microchim Acta 182(11–12):2061–2067
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 268 kb)
Rights and permissions
About this article
Cite this article
Biniaz, Z., Mostafavi, A., Shamspur, T. et al. Electrochemical sandwich immunoassay for the prostate specific antigen using a polyclonal antibody conjugated to thionine and horseradish peroxidase. Microchim Acta 184, 2731–2738 (2017). https://doi.org/10.1007/s00604-017-2284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2284-2