Abstract
This article describes the construction of a sandwich-type of DNA biosensor for detecting a specific DNA sequence of Mycobacterium tuberculosis (MTB). A polyaniline-reduced graphene oxide (PANI-rGO) composite with favorable electrochemical activity was used as a redox nanoprobe for the generation of the voltammetric signal. The composite was decorated with gold nanoparticles (AuNPs) onto which the signal probe was immobilized to form the tracer label. After hybridization between target DNA and tracer label, the voltammetric signal resulting from the polyaniline-reduced graphene oxide (PANI-rGO) redox probe can be apparently observed. The biosensor can detect the specific IS6110 DNA sequence of MTB in the 0.1 pM to 10 nM concentration range, and the detection limit is as low as 50 fM (at an S/N ratio of 3). The biosensor is also highly specific and does not recognize mismatches. It was applied to the determination of denatured PCR products in clinical samples such as sputum, and the results agreed with those obtained by gel electrophoresis. This assay provides a versatile and powerful tool for detection of MTB, and probably for other pathogens if appropriate molecular markers are available.

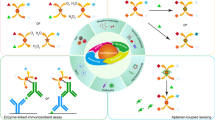
Schematic of an electrochemical DNA biosensor for the ultrasensitive detection of Mycobacterium tuberculosis (MTB). It uses a redox nanoprobe (rGO-PANI) for signal amplification. MCH: 6-mercapto-1-hexanol; PANI: polyaniline; rGO: reduced graphene oxide; rGO-PANI: polyaniline-reduced graphene oxide nanocomposite.






Similar content being viewed by others
Reference
World Health Organization. Global tuberculosis report 2015 [Internet]. Available from: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1. Accessed 06 July 2016
Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM (1999) Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353:444–449
Del Portillo P, Murillo LA, Patarroyo ME (1991) Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol 29:2163–2168
Kumar P, Pandya D, Singh N, Behera D, Aggarwal P, Singh S (2014) Loop-mediated isothermal amplification assay for rapid and sensitive diagnosis of tuberculosis. J Inf Secur 69:607–615
Ng KP, Rukumani DV, Chong J, Kaur H (2014) Identification of Mycobacterium species following growth detection with the BACTEC MGIT 960 system by DNA line probe assay. Int J Mycobacteriol 3:82–87
Izadi Z, Sheikh-Zeinoddin M, Ensafi AA, Soleimanian-Zad S (2016) Fabrication of an electrochemical DNA-based biosensor for Bacillus Cereus detection in milk and infant formula. Biosens Bioelectron 80:582–589
Miodek A, Mejri N, Gomgnimbou M, Sola C, Korri-Youssoufi H (2015) E-DNA sensor of Mycobacterium tuberculosis based on electrochemical assembly of nanomaterials (MWCNTs/PPy/PAMAM). Anal Chem 87:9257–9264
Zhang W, Luo C, Zhong L, Nie S, Cheng W, Zhao D, Ding S (2013) Sensitive detection of enteropathogenic E. coli using a bfpA gene-based electrochemical sensor. Microchim Acta 180:1233–1240
Liu A, Wang K, Weng S, Lei Y, Lin L, Chen W, Lin X, Chen Y (2012) Development of electrochemical DNA biosensors. Trends Anal Chem 37:101–111
Ding L, Bond AM, Zhai J, Zhang J (2013) Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: a review. Anal Chim Acta 797:1–12
Teng Y, Zhang X, Fu Y, Liu H, Wang Z, Jin L, Zhang W (2011) Optimized ferrocene-functionalized ZnO nanorods for signal amplification in electrochemical immunoassay of Escherichia coli. Biosens Bioelectron 26:4661–4666
Wang H, Wang X, Zhang X, Qin X, Zhao Z, Miao Z, Huang N, Chen Q (2009) A novel glucose biosensor based on the immobilization of glucose oxidase onto gold nanoparticles-modified Pb nanowires. Biosens Bioelectron 25:142–146
Dhand C, Das M, Datta M, Malhotra BD (2011) Recent advances in polyaniline based biosensors. Biosens Bioelectron 26:2811–2821
Wnek GE (1986) A proposal for the mechanism of conduction in polyaniline. Synth Met 15:213–218
Tarver J, Yoo JE, Dennes TJ, Schwartz J, Loo Y-L (2009) Polymer acid doped polyaniline is electrochemically stable beyond pH 9. Chem Mater 21:280–286
Hui N, Sun X, Song Z, Niu S, Luo X (2016) Gold nanoparticles and polyethylene glycols functionalized conducting polyaniline nanowires for ultrasensitive and low fouling immunosensing of alpha-fetoprotein. Biosens Bioelectron 86:143–149
Hammond JL, Formisano N, Estrela P, Carrara S, Tkac J (2016) Electrochemical biosensors and nanobiosensors. Essays Biochem 60:69–80
Bai L, Chai Y, Pu X, Yuan R (2014) A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 6:2902–2908
Bai L, Yuan R, Chai Y, Zhuo Y, Yuan Y, Wang Y (2012) Simultaneous electrochemical detection of multiple analytes based on dual signal amplification of single-walled carbon nanotubes and multi-labeled graphene sheets. Biomaterials 33:1090–1096
Gholoobi A, Masoudi-Kazemabad A, Meshkat M, Meshkat Z (2014) Comparison of culture and PCR methods for diagnosis of Mycobacterium tuberculosis in different clinical specimens. Jundishapur J Microbiol 7:e8939
Nghiem MN, Nguyen BV, Nguyen ST, Vo TT, Nong HV (2015) A simple, single triplex PCR of IS6110, IS1081, and 23S ribosomal DNA targets, developed for rapid detection and discrimination of Mycobacterium from clinical samples. J Microbiol Biotechnol 25:745–752
Jana NR, Ying JY (2008) Synthesis of functionalized au nanoparticles for protein detection. Adv Mater 20:430–434
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of au colloid monolayers. Anal Chem 67:735–743
Cannas A, Goletti D, Girardi E, Chiacchio T, Calvo L, Cuzzi G, Piacentini M, Melkonyan H, Umansky SR, Lauria FN, Ippolito G, Tomei LD (2008) Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int J Tuberc Lung Dis 12:146–151
Li L-L, Liu K-P, Yang G-H, Wang C-M, Zhang J-R, Zhu J-J (2011) Fabrication of graphene–quantum dots composites for sensitive Electrogenerated Chemiluminescence Immunosensing. Adv Funct Mater 21:869–878
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys 241:20–22
Huang J, Virji S, Weiller BH, Kaner RB (2003) Polyaniline nanofibers: facile synthesis and chemical sensors. J Am Chem Soc 125:314–315
Silva R, Voiry D, Chhowalla M, Asefa T (2013) Efficient metal-free electrocatalysts for oxygen reduction: polyaniline-derived N- and O-doped mesoporous carbons. J Am Chem Soc 135:7823–7826
Kang ET, Neoh KG, Tan KL (1998) Polyaniline: a polymer with many interesting intrinsic redox states. Prog Polym Sci 23:277–324
Xiang Y, Deng K, Xia H, Yao C, Chen Q, Zhang L, Liu Z, Fu W (2013) Isothermal detection of multiple point mutations by a surface plasmon resonance biosensor with au nanoparticles enhanced surface-anchored rolling circle amplification. Biosens Bioelectron 49:442–449
Li F, Yu Y, Li Q, Zhou M, Cui H (2014) A homogeneous signal-on strategy for the detection of rpoB genes of Mycobacterium tuberculosis based on electrochemiluminescent graphene oxide and ferrocene quenching. Anal Chem 86:1608–1613
Hwang SH, Kim DE, Sung H, Park BM, Cho MJ, Yoon OJ, Lee DH (2015) Simple detection of the IS6110 sequence of Mycobacterium tuberculosis complex in sputum, based on PCR with graphene oxide. PLoS One 10:e0136954
Karaballi RA, Nel A, Krishnan S, Blackburn J, Brosseau CL (2015) Development of an electrochemical surface-enhanced Raman spectroscopy (EC-SERS) aptasensor for direct detection of DNA hybridization. Phys Chem Chem Phys 17:21356–21363
Acknowledgements
This work is supported by National Natural Science Foundation of China (81601856), Funds for Young Science and Technology Talent Cultivation Plan of Chongqing City (cstc2014kjrc-qnrc00004), the National Key Clinical Specialist Construction Programs of China ([2012] No. 649) and Funds for Outstanding Young Scholars in Chongqing Medical University (CYYQ201405).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest exits in the submission of this manuscript, and it is approved by all authors for publication. All procedures performed in this study were in accordance with the ethical standards.
Electronic supplementary material
ESM 1
(DOC 1009 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Li, Y., Yang, Y. et al. A polyaniline-reduced graphene oxide nanocomposite as a redox nanoprobe in a voltammetric DNA biosensor for Mycobacterium tuberculosis . Microchim Acta 184, 1801–1808 (2017). https://doi.org/10.1007/s00604-017-2184-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2184-5