Abstract
Photodynamic therapy (PDT) uses photosensitizers, light and molecular oxygen to generate cytotoxic reactive oxygen species. Its effectiveness is limited to <1 cm due to the limited penetration depth of light. The present study compares the PDT effectivity of the photosensitizer verteporfin (VP) conjugated to gold nanoparticles (AuNPs) (a) by using deeply penetrating X-rays administered in standard radiotherapy doses, and (b) by using red light (690 nm). VP was conjugated to AuNPs of around 12 nm size to enhance the interaction of ionizing radiation with PS. For comparison, VP also was directly exposed to X-rays. It is found that VP alone is stimulated by X-rays to generate singlet oxygen. The conjugate to AuNPs also generated a significant amount of singlet oxygen on irradiation with X-rays in comparison to illumination with 690-nm light. It is also found that the rate of singlet oxygen generation is amplified in case of AuNP-conjugated VP compared to VP alone. The performance of the AuNP-VP conjugate and of the VP alone was tested in Panc 1 cells. Their viability was impaired much more in these two scenarios than with the X-ray radiation only. This suggests excellent perspectives for PDT based on VP and with X-ray stimulation, both as a stand-alone photosensitizer and in Au-NP conjugates. Moreover, both VP and AuNP-VP conjugates show bright fluorescence in physiological media for excitation/emission wavelengths in the range of 405/690 nm; hence they can also be used for simultaneous bioimaging.

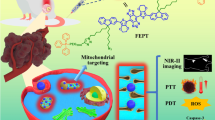
Pancreatic cancer cells (Panc 1) administered with gold nanoparticle- verteporfin conjugate (AuNP-VP) showed an efficient cell killing effect with the generation of singlet oxygen (1O2 ) from triplet oxygen (3O2 ) under 690 nm irradiation and X-ray radiation.







Similar content being viewed by others
References
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q (1998) Photodynamic therapy. J Natl Cancer Inst 90(12):889–905
Macdonald IJ, Dougherty TJ (2001) Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines 5(02):105–129
DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and its applications. Coord Chem Rev 233:351–371
Khaing Oo MK, Yang Y, Hu Y, Gomez M, Du H, Wang H (2012) Gold nanoparticle-enhanced and size- dependent generation of reactive oxygen species from protoporphyrin IX. ACS Nano 6(3):1939–1947
Savarimuthu WP, Gananathan P, Rao AP, Manickam E, Singaravelu G (2015) Protoporphyrin IX-gold nanoparticle conjugates for targeted photodynamic therapy–an In-vitro study. J Nanosci Nanotechnol 15(8):5577–5584
Lucky SS, Soo KC, Zhang Y (2015) Nanoparticles in photodynamic therapy. Chem Rev 115(4):1990–2042
Lucky SS, Muhammad Idris N, Li Z, Huang K, Soo KC, Zhang Y (2015) Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano 9(1):191–205
Jacques SL (2013) Optical properties of biological tissues: a review. Phys Med Biol 58(11):R37
Yu J, Hsu C-H, Huang C-C, Chang P-Y (2014) Development of therapeutic Au–methylene blue nanoparticles for targeted photodynamic therapy of cervical cancer cells. ACS Appl Mater Interfaces 7(1):432–441
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5(3):161–171
Clement S, Sobhan M, Deng W, Camilleri E, Goldys EM (2017) Nanoparticle-mediated singlet oxygen generation from photosensitizers. J Photochem Photobiol A Chem 332:66–71
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65(1):271–284
Li W-T (2009) Nanotechology-based strategies to enhance the efficacy of photodynamic therapy for cancers. Curr Drug Metab 10(8):851–860
da Silva CL, Ciampo D, Orestes J, Rossetti FC, Bentley MVLB, Pierre MBR (2013) PLGA nanoparticles as delivery systems for protoporphyrin IX in topical PDT: cutaneous penetration of photosensitizer observed by fluorescence microscopy. J Nanosci Nanotechnol 13(10):6533–6540
Thienot E, Germain M, Piejos K, Simon V, Darmon A, Marill J, Borghi E, Levy L, Hochepied J-F, Pottier A (2010) One pot synthesis of new hybrid versatile nanocarrier exhibiting efficient stability in biological environment for use in photodynamic therapy. J Photochem Photobiol B Biol 100(1):1–9
Hsu C-Y, Chen C-W, Yu H-P, Lin Y-F, Lai P-S (2013) Bioluminescence resonance energy transfer using luciferase-immobilized quantum dots for self-illuminated photodynamic therapy. Biomaterials 34(4):1204–1212
Wang X, Liu K, Yang G, Cheng L, He L, Liu Y, Li Y, Guo L, Liu Z (2014) Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing. Nanoscale 6(15):9198–9205
Chen H, Wang GD, Chuang Y-J, Zhen Z, Chen X, Biddinger P, Hao Z, Liu F, Shen B, Pan Z (2015) Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett 15(4):2249–2256
Clement S, Deng W, Camilleri E, Wilson BC, Goldys EM (2016) X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: determination of singlet oxygen quantum yield. Sci Rep 6. doi:10.1038/srep19954
El-Sayed IH, Huang X, El-Sayed MA (2006) Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett 239(1):129–135
C-k K, Ghosh P, Rotello VM (2009) Multimodal drug delivery using gold nanoparticles. Nanoscale 1(1):61–67
Yeh Y-C, Creran B, Rotello VM (2012) Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale 4(6):1871–1880
Ashjari M, Dehfuly S, Fatehi D, Shabani R, Koruji M (2015) Efficient functionalization of gold nanoparticles using cysteine conjugated protoporphyrin IX for singlet oxygen production in vitro. RSC Adv 5(127):104621–104628
Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee S-J, Anders RA, Liu JO, Pan D (2012) Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26(12):1300–1305
Manson J, Kumar D, Meenan BJ, Dixon D (2011) Polyethylene glycol functionalized gold nanoparticles: the influence of capping density on stability in various media. Gold Bull 44(2):99–105
Kim S, Fujitsuka M, Majima T (2013) Photochemistry of singlet oxygen sensor green. J Phys Chem B 117(45):13985–13992
Shen Y, Lin H, Huang Z, Xiao L, Chen D, Li B, Xie S (2010) Kinetic analysis of singlet oxygen generation in a living cell using Singlet Oxygen Sensor Green. Proc. SPIE 7845, Optics in Health Care and Biomedical Optics IV, 78451F
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL (1998) One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc 120(9):1959–1964
Shenoy D, Fu W, Li J, Crasto C, Jones G, DiMarzio C, Sridhar S, Amiji M (2006) Surface functionalization of gold nanoparticles using hetero-bifunctional poly (ethylene glycol) spacer for intracellular tracking and delivery. Int J Nanomedicine 1(1):51
Gao J, Huang X, Liu H, Zan F, Ren J (2012) Colloidal stability of gold nanoparticles modified with thiol compounds: bioconjugation and application in cancer cell imaging. Langmuir 28(9):4464–4471
Lee SH, Bae KH, Kim SH, Lee KR, Park TG (2008) Amine-functionalized gold nanoparticles as non-cytotoxic and efficient intracellular siRNA delivery carriers. Int J Pharm 364(1):94–101
Zhang Y, Aslan K, Malyn SN, Geddes CD (2006) Metal-enhanced phosphorescence (MEP). Chem Phys Lett 427(4):432–437
Zhang Y, Aslan K, Previte MJ, Malyn SN, Geddes CD (2006) Metal-enhanced phosphorescence: interpretation in terms of triplet-coupled radiating plasmons. J Phys Chem B 110(49):25108–25114
Mesbahi A (2010) A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep Pract Oncol Radiother 15(6):176–180
Jeremic B, Aguerri AR, Filipovic N (2013) Radiosensitization by gold nanoparticles. Clin Transl Oncol 15(8):593–601
Acknowledgements
The authors thank Mr. Vaughan Moutrie and Mr. Daniel Santos from Genesis Cancer Care for helping us with X-ray radiation experiment. All fluorescence measurements were performed in the Optical Characterization Facility, Linked Laboratory to AMMRF (Australian Microscopy and Microanalysis Research Facility). Authors Sandhya Clement and Wenjie Chen acknowledge the support of an iMQRES scholarship from Macquarie University. This work is partially supported by Australian Research Council (ARC) through its Centre of Excellence scheme (CE140100003) to E.M.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 350 kb)
Rights and permissions
About this article
Cite this article
Clement, S., Chen, W., Anwer, A.G. et al. Verteprofin conjugated to gold nanoparticles for fluorescent cellular bioimaging and X-ray mediated photodynamic therapy. Microchim Acta 184, 1765–1771 (2017). https://doi.org/10.1007/s00604-017-2145-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2145-z