Abstract
A reagentless third generation electrochemical glucose biosensor was fabricated based on wiring the template enzyme glucose oxidase (GOx) with graphene nanoribbons (GN) in order to create direct electron transfer between the co-factor (flavin adenine dinucleotide, FAD) and the electrode. The strategy involved: (i) isolation of the apo-enzyme by separating it from its co-enzyme; (ii) preparation of graphene nanoribbons (GN) by oxidative unzipping of multi-walled carbon nanotubes; (iii) adsorptive immobilization of GNs on the surface of a screen printed carbon electrode (SPCE); (iv) covalent attachment of FAD to the nanoribbons; (v) recombination of the apo-enzyme with the covalently bound FAD to the holoenzyme; and (vi) stabilization of the bio-layer with a thin membrane of Nafion. The biosensor (referred to as GN/FAD/apo-GOx/Nafion/SPCE) is operated at a potential of +0.475 V vs Ag/AgCl/{3 M KCl} in flow-injection mode with an oxygen-free phosphate buffer (pH 7.5) acting as a carrier. The signals are linearly proportional to the concentration of glucose in the range from 50 to 2000 mg⋅L−1 with a detection limit of 20 mg⋅L−1. The repeatability (10 measurements, at 1000 mg⋅L−1 glucose) is ±1.4% and the reproducibility (5 sensors, 1000 mg⋅L−1 glucose) is ±1.8%. The biosensor was applied to the determination of glucose in human serum.

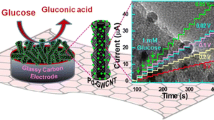
Wiring of the apo-enzyme of glucose oxidase (apo-GOx) with graphene nanoribbons (GN) bound to FAD at a screen-printed carbon electrode (SPCE). Cyclic voltammetric and amperometric responses to various glucose concentrations.




Similar content being viewed by others
References
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814–825. doi:10.1021/cr068123a
Zhang W, Ma D, Du J (2014) Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 120:362–367. doi:10.1016/j.talanta.2013.12.028
Wang C, Huang H (2003) Flow injection analysis of glucose based on its inhibition of electrochemiluminescence in a Ru(bpy)32 + −tripropylamine system. Anal Chim Acta 498:61–68. doi:10.1016/j.aca.2003.08.064
Tian X, Lian S, Zhao L, Chen X, Huang Z, Chen X (2014) A novel electrochemiluminescence glucose biosensor based on platinum nanoflowers/graphene oxide/glucose oxidase modified glassy carbon electrode. J Solid State Electrochem 18:2375–2382. doi:10.1007/s10008-014-2485-0
Mehmeti E, Stankovic DM, Chaiyo S, Svorc L, Kalcher K (2016) Manganese dioxide-modified carbon paste electrode for voltammetric determination of riboflavin. Mikrochimica Acta 183:1619–1624. doi:10.1007/s00604-016-1789-4
Yu J, Liu S, Ju H (2003) Glucose sensor for flow injection analysis of serum glucose based on immobilization of glucose oxidase in titania sol–gel membrane. Biosens Bioelectron 19:401–409. doi:10.1016/S0956-5663(03)00199-4
Turkusic E, Kalcher J, Kahrovic E, Beyene NW, Moderegger H, Sofic E, Begic S, Kalcher K (2005) Amperometric determination of bonded glucose with an MnO(2) and glucose oxidase bulk-modified screen-printed electrode using flow-injection analysis. Talanta 65:559–564. doi:10.1016/j.talanta.2004.07.023
Wong CM, Wong KH, Chen XD (2008) Glucose oxidase: natural occurrence, function, properties and industrial applications. Appl Microbiol Biotechnol 78:927–938. doi:10.1007/s00253-008-1407-4
Wang J (2001) Glucose biosensors: 40 Years of advances and challenges. Electroanalysis 13:983–988
Scheller FW, Schubert F, Neumann B, Pfeiffer D, Hintsche R, Dransfeld I, Wollenberger U, Renneberg R, Warsinke A, Johansson G, Skoog M, Yang X, Bogdanovskaya V, Bückmann A, Zaitsev S (1991) Second generation biosensors. Biosens Bioelectron 6:245–253. doi:10.1016/0956-5663(91)80010-U
Liang B, Fang L, Yang G, Hu Y, Guo X, Ye X (2013) Direct electron transfer glucose biosensor based on glucose oxidase self-assembled on electrochemically reduced carboxyl graphene. Biosens Bioelectron 43:131–136. doi:10.1016/j.bios.2012.11.040
Rakhi RB, Sethupathi K, Ramaprabhu S (2009) A glucose biosensor based on deposition of glucose oxidase onto crystalline gold nanoparticle modified carbon nanotube electrode. J Phys Chem B 113:3190–3194. doi:10.1021/jp810235v
Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I (2003) “Plugging into enzymes”: nanowiring of redox enzymes by a gold nanoparticle. Science 299:1877–1881. doi:10.1126/science.1080664
Liu Y, Wang M, Zhao F, Xu Z, Dong S (2005) The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens Bioelectron 21:984–988. doi:10.1016/j.bios.2005.03.003
Patolsky F, Weizmann Y, Willner I (2004) Long-range electrical contacting of redox enzymes by SWCNT connectors. Angewandte Chemie (International ed in English) 43:2113–2117. doi:10.1002/anie.200353275
Chung H, Chang C, Lin C, Lin M (2016) Electronic and optical properties of graphene nanoribbons in external fields. Physical Chemistry Chemical Physics PCCP 18:7573–7616. doi:10.1039/c5cp06533j
Martín A, Hernández-Ferrer J, Vázquez L, Martínez M, Escarpa A (2014) Controlled chemistry of tailored graphene nanoribbons for electrochemistry: a rational approach to optimizing molecule detection. RSC Adv 4:132–139. doi:10.1039/C3RA44235G
Xiao B, Li X, Li X, Wang B, Langford C, Li R, Sun X (2014) Graphene nanoribbons derived from the unzipping of carbon nanotubes: controlled synthesis and superior lithium storage performance. J Phys Chem C 118:881–890. doi:10.1021/jp410812v
Swoboda B (1969) The relationship between molecular conformation and the binding of flavin-adenine dinucleotide in glucose oxidase. Biochimica et Biophysica Acta (BBA) - Protein Structure 175:365–379. doi:10.1016/0005-2795(69)90014-2
Bückmann AF, Wray V, Stocker A (1997) Synthesis of N6-(2-aminoethyl)-FAD, N6-(6-carboxyhexyl)-FAD, and related compounds. In: Methods in enzymology vitamins and coenzymes part J. Academic Press, p 360–374
Liu J, Chou A, Rahmat W, Paddon-Row M, Gooding J (2005) Achieving direct electrical connection to glucose oxidase using aligned single walled carbon nanotube arrays. Electroanalysis 17:38–46. doi:10.1002/elan.200403116
Taleat Z, Khoshroo A, Mazloum-Ardakani M (2014) Screen-printed electrodes for biosensing: a review (2008–2013). Microchim Acta 181:865–891. doi:10.1007/s00604-014-1181-1
Kochmann S, Hirsch T, Wolfbeis OS (2012) Graphenes in chemical sensors and biosensors. TrAC Trends Anal Chem 39:87–113. doi:10.1016/j.trac.2012.06.004
Devasenathipathy R, Karthik R, Chen S, Ali MA, Mani V, Lou B, Al-Hemaid FMA (2015) Enzymatic glucose biosensor based on bismuth nanoribbons electrochemically deposited on reduced graphene oxide. Microchim Acta 182:2165–2172. doi:10.1007/s00604-015-1545-1
Wang F, Gong W, Wang L, Chen Z (2015) Enhanced amperometric response of a glucose oxidase and horseradish peroxidase based bienzyme glucose biosensor modified with a film of polymerized toluidine blue containing reduced graphene oxide. Microchim Acta 182:1949–1956. doi:10.1007/s00604-015-1535-3
Yang Z, Xu Y, Li J, Jian Z, Yu S, Zhang Y, Hu X, Dionysiou DD (2015) An enzymatic glucose biosensor based on a glassy carbon electrode modified with cylinder-shaped titanium dioxide nanorods. Microchim Acta 182:1841–1848. doi:10.1007/s00604-015-1519-3
Zhang P, Zhang L, Zhao G, Feng F (2012) A highly sensitive nonenzymatic glucose sensor based on CuO nanowires. Microchim Acta 176:411–417. doi:10.1007/s00604-011-0733-x
Wang W, Xie Y, Wang Y, Du H, Xia C, Tian F (2014) Glucose biosensor based on glucose oxidase immobilized on unhybridized titanium dioxide nanotube arrays. Microchim Acta 181:381–387. doi:10.1007/s00604-013-1121-5
Xu S, Qi H, Zhou S, Zhang X, Zhang C (2014) Mediatorless amperometric bienzyme glucose biosensor based on horseradish peroxidase and glucose oxidase cross-linked to multiwall carbon nanotubes. Microchim Acta 181:535–541. doi:10.1007/s00604-014-1175-z
Nien P, Wang J, Chen P, Chen L, Ho K (2010) Encapsulating benzoquinone and glucose oxidase with a PEDOT film: application to oxygen-independent glucose sensors and glucose/O2 biofuel cells. Bioresour Technol 101:5480–5486. doi:10.1016/j.biortech.2010.02.012
Acknowledgements
E. M wishes to acknowledge Higher KOS Stipendien for scholarship support from ADA, MEST and Austrian Agency for International Cooperation in Education and Research (OeAD-GmbH), Centre for International Cooperation & Mobility (ICM). E.M. also thanks Michael Stiboller for help and discussions. S.C. and K.K. appreciate financial mobility support by Asea UniNet. The authors thank Prof. W. Kroutil for very fruitful discussion.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 843 kb)
Rights and permissions
About this article
Cite this article
Mehmeti, E., Stanković, D.M., Chaiyo, S. et al. Wiring of glucose oxidase with graphene nanoribbons: an electrochemical third generation glucose biosensor. Microchim Acta 184, 1127–1134 (2017). https://doi.org/10.1007/s00604-017-2115-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2115-5