Abstract
The authors describe a method for magnetic solid phase extraction of uranyl ions from water samples. It is based on the use of spherical agarose-coated magnetic nanoparticles along with magnetic field agitation. The salen type Schiff base N,N’-bis(4-hydroxysalicylidene)-1,2-phenylenediamine was synthesized from resorcinol in two steps and characterized by infrared and nucleic magnetic resonance spectroscopies. The particles were then activated by an epichlorohydrin method and functionalized with the Schiff base which acts as a selective ligand for the extraction of UO2(II). Following preconcentration and elution with HCl, the ions were quantified by spectrophotometry using Arsenazo III as the indicator. The effects of pH value, ionic strength and amount of the adsorbent on the extraction of UO2(II) were optimized by a multivariate central composite design method. Six replicate analyses under optimized conditions resulted in a recovery of 96.6 % with a relative standard deviation of 3.4 % for UO2(II). The detection limit of the method (at a signal-to-noise ratio of 3σ) is 10 μg L‾1. The method was successfully applied to the determination of UO2(II) in spiked water samples.

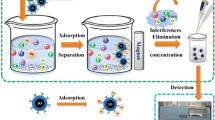
Spherical agarose-coated magnetic nanoparticles (SACMNPs) were prepared in the presence of Span 85 (a nonionic surfactant) and functionalized by a salen type Schiff base for magnetic solid-phase extraction of uranyl ion





Similar content being viewed by others
References
Kalyan Y, Pandey AK, Naidu GRK, Reddy AVR (2009) Membrane optode for uranium(VI) ions preconcentration and quantification based on a synergistic combination of 4-(2-thiazolylazo)-resorcinol with 8-hydroxyquinaldine. Spectrochim Acta A 74:1235–1241. doi:10.1016/j.saa.2009.09.045
Dewar D, Harvey L, Vakil C (2013) Uranium mining and health. Can Fam Physician 59:469–471
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Makelainen I, Vaisanen SB, Penttila IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110:337–342
Kurttio P, Harmoinen A, Saha H, Salonen L, Karpas Z, Komulainen H, Auvinen A (2006) Kidney toxicity of ingested uranium from drinking water. Am J Kidney Dis 47:972–982. doi:10.1053/j.ajkd.2006.03.002
Oshita K, Seo K, Sabarudin A, Oshima M, Takayanagi T, Motomizu S (2008) Synthesis of chitosan resin possessing a phenylarsonic acid moiety for collection/concentration of uranium and its determination by ICP-AES. Anal Bioanal Chem 390:1927–1932. doi:10.1007/s00216-008-1931-1
El Himri M, Pastor A, de la Guardia M (2000) Determination of uranium in tap water by ICP-MS. Fresenius J Anal Chem 367:151–156
Shaw MJ, Hill SJ, Jones P, Nesterenko PN (2000) Determination of uranium in environmental matrices by chelation ion chromatography using a high performance substrate dynamically modified with 2,6-pyridinedicarboxylic acid. Chromatographia 51:695–700. doi:10.1007/BF02505407
Pacheco M, Havel J (2001) Capillary zone electrophoretic (CZE) study of uranium(VI) complexation with humic acids. J Radioanal Nucl Chem 248:565–570. doi:10.1023/A:1010618628705
Khan MH, Warwick P, Evans N (2006) Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 63:1165–1169. doi:10.1016/j.chemosphere.2005.09.060
Niazi A (2006) Spectrophotometric simultaneous determination of uranium and thorium using partial least squares regression and orthogonal signal correction. J Brazil Chem Soc 17:1020–1026
Khan F, Rahman N, Azmi SNH (2009) Spectrophotometric determination of uranium (VI) via complexation with piroxicam. Indian J Chem Tech 16:437–441
Ghiasvand AR, Heidari N, Hashemi P (2014) Highly sensitive and selective determination of uranium in natural waters through a novel solidified floating organic drop microextraction method coupled with spectrophotometric determination. Anal Method 6:5992–5998. doi:10.1039/C4AY00981A
Chandrasekaran K, Karunasagar D, Arunachalam J (2011) Dispersive liquid-liquid micro extraction of uranium(vi) from groundwater and seawater samples and determination by inductively coupled plasma-optical emission spectrometry and flow injection-inductively coupled plasma mass spectrometry. Anal Method 3:2140–2147. doi:10.1039/C1AY05329A
Ghasemi J, Hashemi B, Shamsipur M (2012) Simultaneous spectrophotometric determination of uranium and zirconium using cloud point extraction and multivariate methods. J Iran Chem Soc 9:257–262. doi:10.1007/s13738-011-0019-6
Konstantinou M, Pashalidis I (2004) Speciation and spectrophotometric determination of uranium in seawater. Medit Mar Sci 5:55–60
Dojozan D, Pournaghi-Azar M, Toutounchi-Asr J (1998) Preconcentration of trace uranium from seawater with solid phase extraction followed by differential pulse polarographic determination in chloroform eluate. Talanta 46:123–128
Safarıikova M, Safarıik I (1999) Magnetic solid-phase extraction. J Magn Magn Mater 194:108–112. doi:10.1016/S0304-8853(98)00566-6
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7:1–37. doi:10.1007/BF03245856
Safdarian M, Hashemi P, Adeli M (2013) One-step synthesis of agarose-coated magnetic nanoparticles and their application in the solid phase extraction of Pd(II) using a new magnetic field agitation device. Anal Chim Acta 774:44–50. doi:10.1016/j.aca.2013.03.006
Nazari Serenjeh F, Hashemi P, Rasoolzadeh F (2015) A simple method for the preparation of spherical core–shell nanomagnetic agarose particles. Colloid Surf A 465:47–53. doi:10.1016/j.colsurfa.2014.10.003
Hashemi P, Abolghasemi MM, Alizadeh K, Zarjani RA (2008) A calmagite immobilized agarose membrane optical sensor for selective monitoring of Cu2+. Sens Actuat B 129:332–338. doi:10.1016/j.snb.2007.08.033
Minitab Inc (2013) Minitab statistical software, release 17 for windows. State College, Pennsylvania
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 63.9 kb)
Rights and permissions
About this article
Cite this article
Nazari Serenjeh, F., Hashemi, P., Naeimi, H. et al. Spherical agarose-coated magnetic nanoparticles functionalized with a new salen for magnetic solid-phase extraction of uranyl ion. Microchim Acta 183, 2449–2455 (2016). https://doi.org/10.1007/s00604-016-1882-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1882-8