Abstract
This review (with 90 refs.) covers the state of the art in optofluidic devices with integrated solid-state nanopores for use in detection and sensing. Following an introduction into principles of optofluidics and solid-state nanopore technology, we discuss features of solid-state nanopore based assays using optofluidics. This includes the incorporation of solid-state nanopores into optofluidic platforms based on liquid-core anti-resonant reflecting optical waveguides (ARROWs), methods for their fabrication, aspects of single particle detection and particle manipulation. We then describe the new functionalities provided by solid-state nanopores integrated into optofluidic chips, in particular acting as smart gates for correlated electro-optical detection and discrimination of nanoparticles. This enables the identification of viruses and λ-DNA, particle trajectory simulations, enhancing sensitivity by tuning the shape of nanopores. The review concludes with a summary and an outlook.

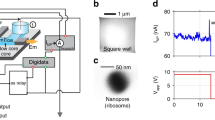
Optical detection of particle translocation through the solid-state nanopore is commonly fullfilled using a top-down microscope. The integration of the solid-state nanopore into the optofluidic chip combines the advantages of solid-state nanopore, optical detection, and fluidics control.






Similar content being viewed by others
References
Stone HA, Stroock AD, Ajdari A (2004) Engineering flows in small devices. Annu Rev Fluid Mech 36:381–411. doi:10.1146/annurev.fluid.36.050802.122124
Squires TM, Quake SR (2005) Microfluidics: fluid physics at the nanoliter scale. Rev Mod Phys 77:977–1026. doi:10.1103/RevModPhys.77.977
Beebe DJ, Mensing GA, Walker GM (2002) Physics and applications of microfluidics in biology. Annu Rev Biomed Eng 4:261–286. doi:10.1146/annurev.bioeng.4.112601.125916
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373. doi:10.1038/nature05058
Dittrich PS, Manz A (2006) Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discov 5:210–218. doi:10.1038/nrd1985
Psaltis D, Quake SR, Yang C (2006) Developing optofluidic technology through the fusion of microfluidics and optics. Nature 442:381–386. doi:10.1038/nature05060
Monat C, Domachuk P, Eggleton BJ (2007) Integrated optofluidics: a new river of light. Nat Photonics 1:106–114. doi:10.1038/nphoton.2006.96
Schmidt H, Hawkins AR (2011) The photonic integration of non-solid media using optofluidics. Nat Photonics 5:598–604. doi:10.1038/nphoton.2011.163
Erickson D, Sinton D, Psaltis D (2011) Optofluidics for energy applications. Nat Photonics 5:583–590. doi:10.1038/nphoton.2011.209
Fan X, White IM (2011) Optofluidic microsystems for chemical and biological analysis. Nat Photonics 5:591–597. doi:10.1038/nphoton.2011.206
Schmidt H, Hawkins AR (2008) Optofluidic waveguides: I. Concepts and implementations. Microfluid Nanofluid 4:3–16
Hawkins AR, Schmidt H (2007) Optofluidic waveguides: II. Fabrication and structures. Microfluid Nanofluid 4:17–32. doi:10.1007/s10404-007-0194-z
Kim S, Streets AM, Lin RR, Quake SR, Weiss S, Majumdar DS (2011) High-throughput single-molecule optofluidic analysis. Nat Methods 8:242–245. doi:10.1038/nmeth.1569
Coskun AF, Cetin AE, Galarreta BC, Alvarez DA, Altug H, Ozcan A (2014) Lensfree optofluidic plasmonic sensor for real-time and label-free monitoring of molecular binding events over a wide field-of-view. Sci Rep 4:6789. doi:10.1038/srep06789
Bertucci A, Manicardi A, Candiani A, Giannetti S, Cucinotta A, Spoto G, Konstantaki M, Pissadakis S, Selleri S, Corradini R (2015) Detection of unamplified genomic DNA by a PNA-based microstructured optical fiber (MOF) Bragg-grating optofluidic system. Biosens Bioelectron 63:248–254. doi:10.1016/j.bios.2014.07.047
Chow E, Grot A, Mirkarimi LW, Sigalas M, Girolami G (2004) Ultracompact biochemical sensor built with two-dimensional photonic crystal microcavity. Opt Lett 29:1093–1095. doi:10.1364/OL.29.001093
Liu P, Huang H, Cao T, Tang Z, Liu X, Qi Z, Ren M, Wu H (2012) An optofluidics biosensor consisted of high-finesse fabry-pérot resonator and micro-fluidic channel. Appl Phys Lett 100:233705. doi:10.1063/1.4726188
Sun Y, Fan X (2011) Optical ring resonators for biochemical and chemical sensing. Anal Bioanal Chem 399:205–211. doi:10.1007/s00216-010-4237-z
Fenzl C, Hirsch T, Wolfbeis OS (2014) Photonic crystals for chemical sensing and biosensing. Angew Chem Int Ed 53:3318–3335. doi:10.1002/anie.201307828
Deng Y-L, Juang Y-J (2013) Electrokinetic trapping and surface enhanced Raman scattering detection of biomolecules using optofluidic device integrated with a microneedles array. Biomicrofluidics 7:014111. doi:10.1063/1.4793224
Song W, Psaltis D (2013) Electrically tunable optofluidic light switch for reconfigurable solar lighting. Lab Chip 13:2708–2713. doi:10.1039/c3lc50204j
Soltani M, Lin J, Forties RA, Inman JT, Saraf SN, Fulbright RM, Lipson M, Wang MD (2014) Nanophotonic trapping for precise manipulation of biomolecular arrays. Nat Nanotechnol 9:448–452. doi:10.1038/nnano.2014.79
Dekker C (2007) Solid-state nanopores. Nat Nanotechnol 2:209–215. doi:10.1038/nnano.2007.27
Stoloff DH, Wanunu M (2013) Recent trends in nanopores for biotechnology. Curr Opin Biotechnol 24:699–704. doi:10.1016/j.copbio.2012.11.008
Bahrami A, Doğan F, Japrung D, Albrecht T (2012) Solid-state nanopores for biosensing with submolecular resolution. Biochem Soc Trans 40:624–628. doi:10.1042/BST20120121
Feng Y, Zhang Y, Ying C, Wang D, Du C (2015) Nanopore-based fourth-generation DNA sequencing technology. Genomics Proteomics Bioinformatics 13:4–16. doi:10.1016/j.gpb.2015.01.009
Miles BN, Ivanov AP, Wilson KA, Doğan F, Japrung D, Edel JB (2012) Single molecule sensing with solid-state nanopores: novel materials, methods, and applications. Chem Soc Rev 42:15–28. doi:10.1039/C2CS35286A
Venkatesan BM, Bashir R (2011) Nanopore sensors for nucleic acid analysis. Nat Nanotechnol 6:615–624. doi:10.1038/nnano.2011.129
Zaino LP, Ma C, Bohn PW (2015) Nanopore-enabled electrode arrays and ensembles. Microchim Acta. doi:10.1007/s00604-015-1701-7
Shi J, Hou J, Fang Y (2015) Recent advances in nanopore-based nucleic acid analysis and sequencing. Microchim Acta:1–15. doi:10.1007/s00604-015-1503-y
Li J, Yu D, Zhao Q (2015) Solid-state nanopore-based DNA single molecule detection and sequencing. Microchim Acta:1–13. doi:10.1007/s00604-015-1542-4
Uram JD, Ke K, Mayer M (2008) Noise and bandwidth of current recordings from submicrometer pores and nanopores. ACS Nano 2:857–872. doi:10.1021/nn700322m
Smeets RMM, Keyser UF, Dekker NH, Dekker C (2008) Noise in solid-state nanopores. Proc Natl Acad Sci 105:417–421. doi:10.1073/pnas.0705349105
Smeets RMM, Dekker NH, Dekker C (2009) Low-frequency noise in solid-state nanopores. Nanotechnology 20:095501. doi:10.1088/0957-4484/20/9/095501
Tabard-Cossa V, Trivedi D, Wiggin M, Jetha NN, Marziali A (2007) Noise analysis and reduction in solid-state nanopores. Nanotechnology 18:305505. doi:10.1088/0957-4484/18/30/305505
Yusko EC, Johnson JM, Majd S, Prangkio P, Rollings RC, Li J, Yang J, Mayer M (2011) Controlling protein translocation through nanopores with bio-inspired fluid walls. Nat Nanotechnol 6:253–260. doi:10.1038/nnano.2011.12
Fologea D, Uplinger J, Thomas B, McNabb DS, Li J (2005) Slowing DNA translocation in a solid-state nanopore. Nano Lett 5:1734–1737. doi:10.1021/nl051063o
Mirsaidov U, Comer J, Dimitrov V, Aksimentiev A, Timp G (2010) Slowing the translocation of double-stranded DNA using a nanopore smaller than the double helix. Nanotechnology 21:395501. doi:10.1088/0957-4484/21/39/395501
Soni GV, Singer A, Yu Z, Sun Y, McNally B, Meller A (2010) Synchronous optical and electrical detection of biomolecules traversing through solid-state nanopores. Rev Sci Instrum 81:014301. doi:10.1063/1.3277116
Chansin GAT, Mulero R, Hong J, Kim MJ, deMello AJ, Edel JB (2007) Single-molecule spectroscopy using nanoporous membranes. Nano Lett 7:2901–2906. doi:10.1021/nl071855d
Kurz V, Nelson EM, Shim J, Timp G (2013) Direct visualization of single-molecule translocations through synthetic nanopores comparable in size to a molecule. ACS Nano 7:4057–4069. doi:10.1021/nn400182s
Auger T, Mathé J, Viasnoff V, Charron G, Di Meglio J-M, Auvray L, Montel F (2014) Zero-mode waveguide detection of flow-driven DNA translocation through nanopores. Phys Rev Lett 113:028302. doi:10.1103/PhysRevLett.113.028302
Larkin J, Foquet M, Turner SW, Korlach J, Wanunu M (2014) Reversible positioning of single molecules inside zero-mode waveguides. Nano Lett 14:6023–6029. doi:10.1021/nl503134x
Anderson BN, Assad ON, Gilboa T, Squires AH, Bar D, Meller A (2014) Probing solid-state nanopores with light for the detection of unlabeled analytes. ACS Nano 8:11836–11845. doi:10.1021/nn505545h
Ivankin A, Henley RY, Larkin J, Carson S, Toscano ML, Wanunu M (2014) Label-free optical detection of biomolecular translocation through nanopore arrays. ACS Nano 8:10774–10781. doi:10.1021/nn504551d
McNally B, Singer A, Yu Z, Sun Y, Weng Z, Meller A (2010) Optical recognition of converted DNA nucleotides for single-molecule DNA sequencing using nanopore arrays. Nano Lett 10:2237–2244. doi:10.1021/nl1012147
Assad ON, Di Fiori N, Squires AH, Meller A (2015) Two color DNA barcode detection in photoluminescence suppressed silicon nitride nanopores. Nano Lett 15:745–752. doi:10.1021/nl504459c
Yukimoto N, Tsutsui M, He Y, Shintaku H, Tanaka S, Kawano S, Kawai T, Taniguchi M (2013) Tracking single-particle dynamics via combined optical and electrical sensing. Sci Rep 3:1855. doi:10.1038/srep01855
Angeli E, Volpe A, Fanzio P, Repetto L, Firpo G, Guida P, Lo Savio R, Wanunu M, Valbusa U (2015) Simultaneous electro-optical tracking for nanoparticle recognition and counting. Nano Lett 15:5696–5701. doi:10.1021/acs.nanolett.5b01243
Datta A, Eom I-Y, Dhar A, Kuban P, Manor R, Ahmad I, Gangopadhyay S, Dallas T, Holtz M, Temkin H, Dasgupta PK (2003) Microfabrication and characterization of Teflon AF-coated liquid core waveguide channels in silicon. IEEE Sensors J 3:788–795. doi:10.1109/JSEN.2003.820343
Wolfe DB, Conroy RS, Garstecki P, Mayers BT, Fischbach MA, Paul KE, Prentiss M, Whitesides GM (2004) Dynamic control of liquid-core/liquid-cladding optical waveguides. Proc Natl Acad Sci U S A 101:12434–12438. doi:10.1073/pnas.0404423101
Risk W, Kim H, Miller R, Temkin H, Gangopadhyay S (2004) Optical waveguides with an aqueous core and a low-index nanoporous cladding. Opt Express 12:6446–6455
Almeida VR, Xu Q, Barrios CA, Lipson M (2004) Guiding and confining light in void nanostructure. Opt Lett 29:1209–1211. doi:10.1364/OL.29.001209
Mandal S, Erickson D (2007) Optofluidic transport in liquid core waveguiding structures. Appl Phys Lett 90:184103. doi:10.1063/1.2735560
Duguay MA, Kokubun Y, Koch TL, Pfeiffer L (1986) Antiresonant reflecting optical waveguides in SiO2-Si multilayer structures. Appl Phys Lett 49:13–15. doi:10.1063/1.97085
Schmidt H, Yin D, Barber JP, Hawkins AR (2005) Hollow-core waveguides and 2-D waveguide arrays for integrated optics of gases and liquids. IEEE J Sel Top Quantum Electron 11:519–527. doi:10.1109/JSTQE.2005.845612
Phillips BS, Jenkins MH, Liu S, Schmidt H, Hawkins AR (2011) Selective thin-film deposition for optofluidic platforms with optimized transmission. IEEE Photon Technol Lett 23:721–723. doi:10.1109/LPT.2011.2132125
Lunt EJ, Wu B, Keeley JM, Measor P, Schmidt H, Hawkins AR (2010) Hollow ARROW waveguides on self-aligned pedestals for improved geometry and transmission. IEEE Photon Technol Lett 22:1147–1149. doi:10.1109/LPT.2010.2051145
Zhao Y, Jenkins M, Measor P, Leake K, Liu S, Schmidt H, Hawkins AR (2011) Hollow waveguides with low intrinsic photoluminescence fabricated with Ta2O5 and SiO2 films. Appl Phys Lett 98:091104. doi:10.1063/1.3561749
Holmes MR, Liu S, Keeley J, Jenkins M, Leake K, Schmidt H, Hawkins AR (2011) Hollow waveguides with low intrinsic photoluminescence fabricated with PECVD silicon nitride and silicon dioxide films. IEEE Photon Technol Lett 23:1466–1468. doi:10.1109/LPT.2011.2162625
Measor P, Phillips BS, Chen A, Hawkins AR, Schmidt H (2011) Tailorable integrated optofluidic filters for biomolecular detection. Lab Chip 11:899–904. doi:10.1039/c0lc00496k
Ozcelik D, Phillips BS, Parks JW, Measor P, Gulbransen D, Hawkins AR, Schmidt H (2012) Dual-core optofluidic chip for independent particle detection and tunable spectral filtering. Lab Chip 12:3728–3733. doi:10.1039/c2lc40700k
Yin D, Deamer DW, Schmidt H, Barber JP, Hawkins AR (2006) Single-molecule detection sensitivity using planar integrated optics on a chip. Opt Lett 31:2136–2138
Yin D, Lunt EJ, Barman A, Hawkins AR, Schmidt H (2007) Microphotonic control of single molecule fluorescence correlation spectroscopy using planar optofluidics. Opt Express 15:7290–7295
Yin D, Lunt EJ, Rudenko MI, Deamer DW, Hawkins AR, Schmidt H (2007) Planar optofluidic chip for single particle detection, manipulation, and analysis. Lab Chip 7:1171–1175. doi:10.1039/B708861B
Rudenko MI, Kühn S, Lunt EJ, Deamer DW, Hawkins AR, Schmidt H (2009) Ultrasensitive Qβ phage analysis using fluorescence correlation spectroscopy on an optofluidic chip. Biosens Bioelectron 24:3258–3263. doi:10.1016/j.bios.2009.04.005
Chen A, Eberle MM, Lunt EJ, Liu S, Leake K, Rudenko MI, Hawkins AR, Schmidt H (2011) Dual-color fluorescence cross-correlation spectroscopy on a planar optofluidic chip. Lab Chip 11:1502–1506. doi:10.1039/c0lc00401d
Kühn S, Lunt EJ, Phillips BS, Hawkins AR, Schmidt H (2009) Optofluidic particle concentration by a long-range dual-beam trap. Opt Lett 34:2306–2308
Kühn S, Measor P, Lunt EJ, Phillips BS, Deamer DW, Hawkins AR, Schmidt H (2009) Loss-based optical trap for on-chip particle analysis. Lab Chip 9:2212–2216. doi:10.1039/b900555b
Kühn S, Phillips BS, Lunt EJ, Hawkins AR, Schmidt H (2010) Ultralow power trapping and fluorescence detection of single particles on an optofluidic chip. Lab Chip 10:189–194. doi:10.1039/b915750f
Leake KD, Phillips BS, Yuzvinsky TD, Hawkins AR, Schmidt H (2013) Optical particle sorting on an optofluidic chip. Opt Express 21:32605–32610. doi:10.1364/OE.21.032605
Leake KD, Olson MAB, Ozcelik D, Hawkins AR, Schmidt H (2015) Spectrally reconfigurable integrated multi-spot particle trap. Opt Lett 40:5435–5438. doi:10.1364/OL.40.005435
Parks JW, Cai H, Zempoaltecatl L, Yuzvinsky TD, Leake K, Hawkins AR, Schmidt H (2013) Hybrid optofluidic integration. Lab Chip 13:4118–4123. doi:10.1039/C3LC50818H
Cai H, Parks JW, Wall TA, Stott MA, Stambaugh A, Alfson K, Griffiths A, Mathies RA, Carrion R, Patterson JL, Hawkins AR, Schmidt H (2015) Optofluidic analysis system for amplification-free, direct detection of ebola infection. Sci Rep 5:14494. doi:10.1038/srep14494
Ozcelik D, Parks JW, Wall TA, Stott MA, Cai H, Parks JW, Hawkins AR, Schmidt H (2015) Optofluidic wavelength division multiplexing for single-virus detection. Proc Natl Acad Sci 112:12933–12937. doi:10.1073/pnas.1511921112
Holmes MR, Shang T, Hawkins AR, Rudenko M, Measor P, Schmidt H (2010) Micropore and nanopore fabrication in hollow antiresonant reflecting optical waveguides. J Micronanolithography MEMS MOEMS JM3 9:023004. doi:10.1117/1.3378152
Zhao Q, Sigalov G, Dimitrov V, Dorvel B, Mirsaidov U, Sligar S, Aksimentiev A, Timp G (2007) Detecting SNPs using a synthetic nanopore. Nano Lett 7:1680–1685. doi:10.1021/nl070668c
Matysiak S, Montesi A, Pasquali M, Kolomeisky AB, Clementi C (2006) Dynamics of polymer translocation through nanopores: theory meets experiment. Phys Rev Lett 96:118103. doi:10.1103/PhysRevLett.96.118103
Ghosal S (2007) Electrokinetic-flow-induced viscous drag on a tethered DNA inside a nanopore. Phys Rev E 76:061916. doi:10.1103/PhysRevE.76.061916
Tian P, Smith GD (2003) Translocation of a polymer chain across a nanopore: a Brownian dynamics simulation study. J Chem Phys 119:11475–11483. doi:10.1063/1.1621614
Meller A, Nivon L, Branton D (2001) Voltage-driven DNA translocations through a nanopore. Phys Rev Lett 86:3435–3438. doi:10.1103/PhysRevLett.86.3435
Liu S, Yuzvinsky TD, Schmidt H (2013) Effect of fabrication-dependent shape and composition of solid-state nanopores on single nanoparticle detection. ACS Nano 7:5621–5627. doi:10.1021/nn4020642
Davenport M, Healy K, Pevarnik M, Teslich N, Cabrini S, Morrison AP, Siwy ZS, Létant SE (2012) The role of pore geometry in single nanoparticle detection. ACS Nano 6:8366–8380. doi:10.1021/nn303126n
Liu S (2014) Electro-opto-fluidics: nanopore-gated devices for multimodal analysis of single biomolecules. UNIVERSITY OF CALIFORNIA, SANTA CRUZ
Rudenko MI, Holmes MR, Ermolenko DN, Lunt EJ, Gerhardt S, Noller HF, Deamer DW, Hawkins A, Schmidt H (2011) Controlled gating and electrical detection of single 50S ribosomal subunits through a solid-state nanopore in a microfluidic chip. Biosens Bioelectron 29:34–39. doi:10.1016/j.bios.2011.07.047
Liu S, Zhao Y, Parks JW, Deamer DW, Hawkins AR, Schmidt H (2014) Correlated electrical and optical analysis of single nanoparticles and biomolecules on a nanopore-gated optofluidic chip. Nano Lett 14:4816–4820. doi:10.1021/nl502400x
Liu S, Wall TA, Ozcelik D, Parks JW, Hawkins AR, Schmidt H (2015) Electro-optical detection of single λ-DNA. Chem Commun 51:2084–2087. doi:10.1039/C4CC07591A
Heng JB, Aksimentiev A, Ho C, Marks P, Grinkova YV, Sligar S, Schulten K, Timp G (2005) Stretching DNA using the electric field in a synthetic nanopore. Nano Lett 5:1883–1888. doi:10.1021/nl0510816
Wu L, Liu H, Zhao W, Wang L, Hou C, Liu Q, Lu Z (2014) Electrically facilitated translocation of protein through solid nanopore. Nanoscale Res Lett 9:140. doi:10.1186/1556-276X-9-140
Wanunu M, Morrison W, Rabin Y, Grosberg AY, Meller A (2010) Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient. Nat Nanotechnol 5:160–165. doi:10.1038/nnano.2009.379
Acknowledgments
We thank D.W. Deamer and H.F. Noller for fruitful discussions, T.D. Yuzvinsky for EDS spectroscopy analysis, Y. Zhao and T.A. Wall for ARROW chips fabrication, D. Ozcelik for particle trajectory simulations, and J.W. Parks for virus sample preparation. We acknowledge support by the W.M. Keck Center for Nanoscale Optofluidics at University of California, Santa Cruz, the NSF under grants CBET-1402848 and CBET-1159423, and the NIH under grants R01EB006097 and R21EB008802.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Hawkins, A.R. & Schmidt, H. Optofluidic devices with integrated solid-state nanopores. Microchim Acta 183, 1275–1287 (2016). https://doi.org/10.1007/s00604-016-1758-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1758-y