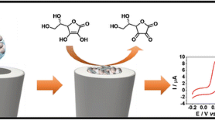
Abstract
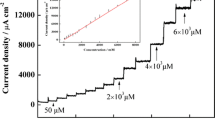
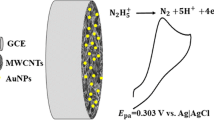
This article reports on an electrochemical sensor for thiourea. It is based on a glassy carbon electrode (GCE) modified with a self-assembled monolayer of an oxadiazole derivative and with silver nanoparticles. The modified GCE demonstrated highly catalytic activity in terms of thiourea oxidation. The peak potential is shifted to negative values compared to a GCE coated with silver nanoparticles only. The electrode was characterized by linear sweep voltametry, cyclic voltammetry and chronoamperometry, and thiourea was determined by differential pulse voltammetry in aqueous buffer of pH 7.0 resulting in two linear response ranges of 0.001 − 69.4 and 69.4 − 833.3 μM and the limit of detection of 0.1 nM. The method was applied to the determination of thiourea in copper refinery electrolyte, orange juice and tap water samples. The recoveries ranged from 96.9 to 108.0 %.

A glassy carbon electrode was modified with silver nanoparticles (SNP−GCE) using continuous cyclic potential in solution of nitric acid and AgNO3. Oxadiazole - modified SNP−GCE (OSNP−GCE) was prepared by the self-assembling technique directly onto the SNP−GCE.







Similar content being viewed by others
References
Krzewska S, Pajdowski L, Podsiadly H, Podsiadly J (1984) Electrochemical determination of thiourea and glue in the industrial copper electrolyte. Metall Trans 15:451–459
Suarez D, Olson F (1992) Nodulation of electrodeposited copper in the presence of thiourea. J Appl Electrochem 22:1002–1010
Abbasi S, Khani H, Hosseinzadeh L, Safari Z (2010) Determination of thiourea in fruit juice by a kinetic spectrophotometric method. J Hazard Mater 174:257–262
Hoseney R, Finney K (1964) Spectrophotometric determination of urea, thiourea, and certain of their substitution products with p-dimethylaminobenzaldehyde and diacetylmonoxime. Anal Chem 36:2145–2148
Mandrou B, Brun S, Kingkate A (1977) Quantitative determination of thiourea in citrus fruits. J Assoc Off Ana Chem 60:699–701
Kargosha K, Khanmohammadi M, Ghadiri M (2001) Fourier transform infrared spectrometric determination of thiourea in the presence of sulphur dioxide in aqueous solution. Anal Chim Acta 437:139–143
Kargosha K, Khanmohammadi M, Ghadiri M (2001) Vapour phase fourier transform infrared spectrometric determination of thiourea. Analyst 126:1432–1435
Li WH, Wang YZ (2006) Determination of thiourea by flow injection with chemiluminescence. Physical testing and chemical analysis (Part B: Chemical Analysis) 42:111
Fan X, Wang S, Chen F, Wang X (2012) FI-Chemiluminescence determination of thiourea [J]. Physical Testing and Chemical Analysis (Part B: Chemical Analysis) 12:31
Bowley HJ, Crathorne EA, Gerrard DL (1986) Quantitative determination of thiourea in aqueous solution in the presence of sulphur dioxide by Raman spectroscopy. Analyst 111:539–542
Rethmeier J, Neumanna G, Stumpf C, Rabenstein A, Vogt C (2001) Determination of low thiourea concentrations in industrial process water and natural samples using reversed-phase high-performance liquid chromatography. J Chromatogr A 934:129–134
Li X, Wang J, Zhao Z, Wu G, Pang Y (2009) Determination of thiourea in flour by high performance liquid chromatography [J]. Sci Technol Food Ind 5:99
Stara V, Kopanica M (1984) Adsorptive stripping voltammetric determination of thiourea and thiourea derivatives. Anal Chim Acta 159:105–110
Pápay M, Tóth K, Pungor E (1971) Potentiometric determination of thiourea with a sulphide-selective membrane electrode. Anal Chim Acta 56:291–296
Akeneev YA, Zakharova EA, Slepchenko GB, Pikula NP (2005) Voltammetric determination of thiourea in copper refinery electrolytes. J Anal Chem 60:514–517
Spataru N, Spataru T, Fujishima A (2005) Voltammetric determination of thiourea at conductive diamond electrodes. Electroanal 17:800–805
Tian L, Gao Y, Li L, Wu W, Sun D, Lu J, Li T (2013) Determination of thiourea using a carbon paste electrode decorated with copper oxide nanoparticles. Microchim Acta 180:607–612
Corb I, Manea F, Radovan C, Pop A, Burtica G, Malchev P, Picken S, Schoonman J (2007) Carbon-based composite electrodes: preparation, characterization and application in electroanalysis. Sensors 7:2626–2635
Levent A, Keskin E, Yardım Y, Şentürk Z (2011) Electrooxidation of thiourea and its square-wave voltammetric determination using pencil graphite electrode. Rev Anal Chem 30:45–51
Sezgintürk MK, Dinçkaya E (2010) Development of a biosensor for controlling of thiourea in fruit juices. Food Bioprocess Tech 3:128–134
Ríos A, Zougagh M, Bouri M (2013) Magnetic (nano) materials as an useful tool for sample preparation in analytical methods. A review. Anal Methods 5:4558–4573
Khodaveisi J, Dadfarnia S, Haji Shabani AM, Rohani Moghadam M, Hormozi-Nezhad MR (2015) Artificial neural network assisted kinetic spectrophotometric technique for simultaneous determination of paracetamol and p-aminophenol in pharmaceutical samples using localized surface plasmon resonance band of silver nanoparticle. Spectrochim Acta A 138:474–480
Nasirizadeh N, Aghayizadeh MM, Bidoki M, Yazdanshenas ME (2013) A novel sensor of quinazolin derivative self-assembled monolayers over silver nanoparticle for the determination of hydroxylamine. Int J Electrochem Sci 8:11264–11277
Goyal RN (2015) Gold nanoparticles decorated poly-melamine modified glassy carbon sensor for the voltammetric estimation of domperidone in pharmaceuticals and biological fluids. Talanta 141:53–59
Van Hieu N, Duc NAP, Trung T, Tuan MA, Chien ND (2010) Gas-sensing properties of tin oxide doped with metal oxides and carbon nanotubes: a competitive sensor for ethanol and liquid petroleum gas. Sensor Actuat B-Chem 144:450–456
Narang J, Chauhan N, Jain P, Pundir CS (2012) Silver nanoparticle/multiwalled carbon nanotube/polyaniline film for amperometric glutathione biosensor. Int J Biol Macromol 50:672–678
Wang G, Wang W, Wu J, Liu H, Jiao S, Fang B (2009) Self-assembly of a silver nanoparticle modified electrode and its electrocatalysis on neutral red. Microchim Acta 164:149–155
Fakhari AR, Saeed Hosseiny Davarani S, Ahmar H, Hasheminasab K, Khavasi HR (2009) A facile electrochemical method for the synthesis of 5-phenyl-1,3,4-oxadiazol-2-ylthio-benzene-1,2-diol derivatives. J Heterocyclic Chem 46:443–446
Ju H, Shen C (2001) Electrocatalytic reduction and determination of dissolved oxygen at a poly (nile blue) modified electrode. Electroanal 13:789–793
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interfacial Electrochem 101:19–28
Nasirizadeh N, Zare HR (2009) Differential pulse voltammetric simultaneous determination of noradrenalin and acetaminophen using a hematoxylin biosensor. Talanta 80:656–663
Andrieux CP, Saveant JM (1978) Heterogeneous (chemically modified electrodes, polymer electrodes) vs. homogeneous catalysis of electrochemical reactions. J Electroanal Chem Interfacial Electrochem 93:163–168
Bard AJ, Faulkner LR (2001) Fundamentals and applications. Electrochemical methods, 2nd edn. Wiley, New York
Abbasi S, Khani H, Bagher Gholivand N, Naghipour A, Farmany A, Abbasi F (2009) A kinetic method for the determination of thiourea by its catalytic effect in micellar media. Spectrochim Acta A 72:327–331
de Oliveira AN, de Santana H, Zaia HCTBV, Zaia DAM (2004) A study of reaction between quinones and thiourea: determination of thiourea in orange juice. J Food Compos Anal 17:165–177
Kurzawa JK, Janowicz K (2005) Used of stopped-flow technique for investigation and determination of thiourea and its N-methyl derivatives as inducer for the iodine-azide reaction. Anal Biol Chem 382:1584–1589
Arab Chamjangali M, Goudarzi, Ghochani Moghadam A, Amin AH (2015) An on-line spectrophotometric determination of trace amounts of thiourea in tap water, orange juice, and orange peel samples using multi-channel flow injection analysis. Spectrochim Acta A 149:580–587
Zhang C, Jiang Y, Zhang D, Shan Y, Yang G (2015) Electrochemical determination of thiourea using thiophene derivatives-modified glassy carbon electrodes. ECS Electrochem Lett 4:B1–B3
Manea F, Radovan C, Schoonman J (2006) Amperometric determination of thiourea in alkaline media on a copper oxide–copper electrode. J Appl Electrochem 36:1075–1081
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Rohani Moghadam, M., Akbarzadeh, S. & Nasirizadeh, N. Electrochemical sensor for the determination of thiourea using a glassy carbon electrode modified with a self-assembled monolayer of an oxadiazole derivative and with silver nanoparticles. Microchim Acta 183, 1069–1077 (2016). https://doi.org/10.1007/s00604-015-1723-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1723-1