Abstract
The authors report that the cationic polymer-oligomer poly(hexamethylene guanidine) (PHMG) in the form of its hydrochloride induces the formation of mixed aggregates composed of anionic magnetiс nanoparticles (magNPs), PHMG and anionic quantum dots (QDs). The magNPs consisted of polymer-coated magnetite nanoparticles, and the QDs consisted of polymer-coated CdSe@CdS/ZnS nanoparticles with an emission maximum at 617 nm. This finding is exploited in a semi-quantitative method for the determination of PHMG. The protocol includes magnetic separation of the mixed aggregates (magNPs/PHMG/QDs) from the sample and excess QDs, redispersion of the aggregates in water, and measurement of fluorescence intensity. The signal is proportional to the concentration of PHMG in the 0.05 to 0.2 mg L−1 concentration range, with intra-day RSDs of up to 27 %. The limit of detection (LOD) of PHMG in spiked run-off waters, swimming pool water and wastewater is 23 μg L−1. This PHMG assay is selective in that high concentrations of surfactants and inorganic salts are tolerated. Polyethyleneimine and poly(diallyldimethylammonium chloride) also cause the formation of mixed aggregates but only at higher concentrations. Both a fluorometer and a digital camera (using a 365-nm LED as a light source) were used to measure fluorescence. In case of using a digital camera, the LOD is 40 μg L−1 and the intraday RSDs are up to 23 %. The method is sensitive, fairly selective and rather simple.

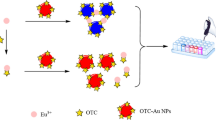
Cationic polymer binds anionic quantum dots and anionic magnetic nanoparticles; mixed aggregates are thus formed. After magnetic separation from the excess of quantum dots, their fluorecence is measured.





Similar content being viewed by others
References
Oulé MK, Quinn K, Dickman M, Bernier AM, Rondeau S, Moissac DD, Boisvert A, Diop L (2012) Akwaton, polyhexamethylene-guanidine hydrochloride-based sporicidal disinfectant: a novel tool to fight bacterial spores and nosocomial infections. J Med Microbiol 61:1421–1427
Antonik LM, Barkova NP, Khabibulina AG, Lopyrev VA (2009) Synthesis and antiseptic and antidote activity of polyhexamethyleneguanidine hydroxyethylidenediphosphonate salt. Pharm Chem J 43:84–86, 0091.150X/09/4302.0084
Krotov YuA, Karelin AO, Loit AO et al. (2000) Mir i Semya, St.-Petersburg (in Russian). Maximum allowable concentrations of chemicals in the environment. Handbook. Ed. See also http://meganorm.ru/Data2/1/4293841/4293841858.htm (accessed September, 2015).
Efimov KM, Danilina NO, Ovcharenko EO et al (2004) Method for quantitative determination of the concentration of polyhexamethyleneguanidine hydrochloride in water. Russ Pat No 2252413. http://www.findpatent.ru/patent/225/2252413.html (accessed September, 2015)
Chmilenko TS, Galimbievsky EA, Chmilenko FA et al (2010) Formation of bromophenol red ion associates and their interaction with polyhexamethyleneguanidine in aqueous solutions. Methods Objects Chem Anal 5:19–28, http://www.moca.net.ua/10/pdf/010510-19-28.pdf. Accessed September, 2015
Slepchenko GB, Moiseeva ES, Khazanov VA et al. (2008) Method for quantitative determination of Anavidin by stripping voltammetry, Russ. Pat. No 2381501. http://www.freepatent.ru/patents/2381501. Accessed September, 2015
Apyari VV, Dmitrienko SG, Zolotov YuA et al. (2011) A method for determining polyhexamethyleneguanidine hydrochloride, Russ. Pat. No 2460998 http://www.freepatent.ru/patents/2460998. Accessed September, 2015
Arkhipova VV, Apyari VV, Dmitrienko SG (2015) Determination of polyhexamethyleneguanidine hydrochloride using gold nanoparticles and polyurethane foam. Moscow Univ Chem Bull 70:28–33. doi:10.3103/S0027131415010022
Artemyeva AA, Samarina TO, Sharov AV, Abramchuk SS, Ovcharenko EO, Dityuk AI, Efimov KM, Beklemishev MK (2015) Highly sensitive determination of poly(hexamethylene guanidine) by rayleigh scattering using aggregation of silver nanoparticles. Microchim Acta 182:965–973. doi:10.1007/s00604-014-1411-6
Kaur R, Hasan A, Iqbal N, Alam S, Saini MK, Raza SK (2014) Synthesis and surface engineering of magnetic nanoparticles for environmental cleanup and pesticide residue analysis: a review. J Sep Sci 37:1805–1825. doi:10.1002/jssc.201400256
Xie L, Jiang R, Zhu F, Liu H, Ouyang G (2014) Application of functionalized magnetic nanoparticles in sample preparation. Anal Bioanal Chem 406:377–399. doi:10.1007/s00216-013-7302-6
Tennico HY, Hutanu D, Koesdjojo MT, Bartel CM, Remcho VT (2010) On-chip aptamer-based sandwich assay for thrombin detection employing magnetic beads and quantum dots. Anal Chem 82:5591–5597. doi:10.1021/ac101269u
Bruno JG, Richarte AM, Phillips T, Savage AA, Sivils JC, Greis A, Mayo MW (2014) Development of a fluorescent enzyme-linked DNA aptamer-magnetic bead sandwich assay and portable fluorometer for sensitive and rapid leishmania detection in sandflies. J Fluoresc 24:267–277. doi:10.1007/s10895-013-1315-6
Krejcova L, Hynek D, Guran R, Michalek P, Moulick A, Kopel P, Tmejova K, Hoai NV, Adam V, Hubalek J (2014) Beads based electrochemical assay for detection of hemagglutinin labeled by two different types of cadmium quantum dots. Int J Electrochem Sci 9:3349–3363, http://www.electrochemsci.org/papers/vol9/90703349.pdf
Uddayasankar U, Zhang Z, Shergill RT, Gradinaru CC, Krull UJ (2014) Isolation of monovalent quantum dot – nucleic acid conjugates using magnetic beads. Bioconjug Chem 25:1342–1350. doi:10.1021/bc5002032
Sun H, Wang M, Wang J, Tian M, Wang H, Sun Z, Huang P (2015) Development of magnetic separation and quantum dots labeled immunoassay for the detection of mercury in biological samples. J Trace Elem Med Biol 30:37–42. doi:10.1016/j.jtemb.2015.01.009
Yang H, Qin L, Wang Y, Zhang B, Liu Z, Ma H, Lu J, Huang X, Shi D, Hu Z (2015) Detection of Mycobacterium tuberculosis based on H37Rv binding peptides using surface functionalized magnetic microspheres coupled with quantum dots—a nano detection method for Mycobacterium tuberculosis. Int J Nanomed 10:77–88. doi:10.2147/IJN.S71700
Hwang HJ, Nam JJ, Yang SI, Kwon J-H, Oh HB (2013) MALDI-TOF analysis of polyhexamethylene guanidine (PHMG) oligomers used as a commericial antibacterial humidifier disinfectant. Bull Korean Chem Soc 34:1708–1714. doi:10.5012/bkcs.2013.34.6.1708
Massart R (1981) Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn 17:1247–1248. doi:10.1109/TMAG.1981.1061188
Smith AM, Duan H, Rhyner MN, Ruan G, Nie S (2006) A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys Chem Chem Phys 8:3895–3903. doi:10.1039/B606572B
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 50:4738–4743. doi:10.1016/j.carbon.2012.06.002
Gembitsky PA (2000) Eco-friendly polymer biocides. materials and technologies. (Ekologichesky bezopasnye polimernye biotsidy. Materialy i tekhnologii.) Collection of articles. Moscow (1) 5
Methods for the determination of the residual concentration of disinfectant “DeFlok” in water (by PHMG-HCl). http://deflok.ru/wp-content/uploads/2013/05/DeFlok_Prilozhenie.pdf. Accessed 30 October, 2015
Rudnev AV, Dzherayan TG (2006) Determination of polyhexamethyleneguanidine by capillary electrophoresis. J Analyt Chem 61:1002–1005. doi:10.1134/S1061934806100091
Chmilenko FA, Korobova IV, Mikulenko OV (2008) Potentiometric sensors for the determination of water-soluble polyelectrolytes. J Analyt Chem 63:590–595. doi:10.1134/S1061934808060130
Acknowledgments
Authors thank Russian Science Foundation for the financial support (grant No 14-23-00012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 721 kb)
Rights and permissions
About this article
Cite this article
Likhachev, K.V., Ovcharenko, E.O., Dityuk, A.I. et al. Fluorescent determination of poly(hexamethylene guanidine) via the aggregates it forms with quantum dots and magnetic nanoparticles. Microchim Acta 183, 1079–1087 (2016). https://doi.org/10.1007/s00604-015-1720-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1720-4