Abstract
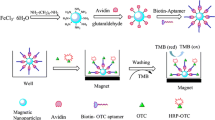
This work describes a method for the simultaneous detection of oxytetracycline (OTC) and kanamycin (KMY) using aptamers acting as both recognition and separation elements, and complementary oligonucleotides labeled with a green emitting fluorophore (carboxyfluorescein, FAM) and a yellow emitting fluorophore (carboxy-X-rhodamine, ROX), respectively, as signal labels. An OTC aptamer and a KMY aptamer were immobilized on the surface of magnetic nanoparticles (MNPs) via avidin-biotin chemistry. The aptamers preferentially bind their respective targets and thereby cause the upconcentration of analytes. However, in their absence they bind fluorescently-tagged complementary oligonucleotide later added to the reaction system. This cause the NPs to become fluorescent, with emission peaks located at 520 and 608 nm, respectively. The effects of the concentration of avidin, aptamer, complementary oligonucleotide, incubation temperature and incubation time were optimized. Under the optimal conditions, linear relationships were obtained in the range of 1–50 ng∙mL−1 for OTC and KMY, with limits of detection of 0.85 ng∙mL−1 and 0.92 ng∙mL−1, respectively. The method was applied to the analysis of pork, milk, and honey samples spiked with OTC and MKY. Recoveries ranged from 76.5 to 94.7 % and 77.8 to 93.1 %, respectively, and the relative standard deviation was <10.0 %.

This work describes an assay for the simultaneous detection of oxytetracycline and kanamycin using aptamer-modified as both recognition and separation elements, and complementary oligonucleotide labeled with FAM and ROX, respectively, as signal labels. The developed method possesses high sensitivity and selectivity, and short analysis time.





Similar content being viewed by others
References
De Wasch K, Okerman L, Croubels S, De Brabander H, Van Hoof J, De Backer P (1998) Detection of residues of tetracycline antibiotics in pork and chicken meat: correlation between results of screening and confirmatory tests. Analyst 123: 2737–2741.
Furusawa N (1999) Spiramycin, oxytetracycline and sulphamonomethoxine contents of eggs and egg-forming tissues of laying hens. J Vet Med A 46: 599–603.
Fritz JW, Zuo YG (2007) Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography. Food Chem 105: 1297–1301
Pérez-Silva I, Rodríguez JA, Ramírez-Silva MT, Páez-Hernández ME (2012) Determination of oxytetracycline in milk samples by polymer inclusion membrane separation coupled to high performance liquid chromatography. Anal Chim Acta 718: 42–46
Gbylik-Sikorska M, Posyniak A, Sniegocki T, Zmudzki J (2015) Liquid chromatography–tandem mass spectrometry multiclass method for the determination of antibiotics residues in water samples from water supply systems in food-producing animal farms. Chemosphere 119: 8–15.
Bimazubute MA, Rozet E, Dizier I, Heugen JCV, Arancio E, Gustin P, Crommen J, Chiap P (2009) Pre-study and in-study validation of an ultra-high pressure LC method coupled to tandem mass spectrometry for off-line determination of oxytetracycline in nasal secretions of healthy pigs. J Chromatogr B 877: 2349–2357.
Herrera-Herrera AV, Ravelo-Pérez LM, Hernández-Borges J, Afonso MM, Palenzuela JA, Rodríguez-Delgado MÁ (2011) Oxidized multi-walled carbon nanotubes for the dispersive solid-phase extraction of quinolone antibiotics from water samples using capillary electrophoresis and large volume sample stacking with polarity switching. J Chromatogr A 1218: 5352–5361.
Sierra-Rodero M, Fernández-Romero JM, Gómez-Hens A (2014) Determination of fluoroquinolone antibiotics by microchip capillary electrophoresis along with time-resolved sensitized luminescence of their terbium(III) complexes. Microchim Acta 181: 1897–1904
Rawat KA, Basu H, Singhl RK, Kailasa SK (2015) Simultaneous colorimetric detection of four drugs in their pharmaceutical formulations using unmodified gold nanoparticles as a probe. RSC Adv 5: 19924–19932.
Rawat KA, Surati KR, Kailasa SK (2014) One-pot synthesis of gold nanoparticles by using 4-aminoantipyrine as a novel reducing and capping agent for simultaneous colorimetric sensing of four triptan-family drugs. Anal Methods 6: 5972–5980.
Laliwala SK, Mehta VN, Rohit JV, Kailasa SK (2014) Citrate-modified silver nanoparticles as a colorimetric probe forsimultaneous detection of four triptan-family. Sensor Actuat B-Chem 197: 254–263.
Galarini R, Diana F, Moretti S, Puppini B, Saluti G, Persic L (2014) Development and validation of a new qualitative ELISA screening for multiresidue detection of sulfonamides in food and feed. Food Control 35: 300–310.
Byzova NA, Smirnova NI, Zherdev AV, Eremin SA, Shanin IA, Lei HT, Sun YM, Dzantiev BB (2014) Rapid immunochromatographic assay for ofloxacin in animal original foodstuffs using native antisera labeled by colloidal gold. Talanta 119: 125–132.
Yuan Q, Lu DQ, Zhang XB, Chen Z, Tan WH (2012) Aptamer-conjugated optical nanomaterials for bioanalysis. Trac-Trend Anal Chem 39: 72–86.
Song KM, Cho M, Jo H, Min K, Jeon SH, Kim T, Han MS, Ku JK, Ban C (2011) Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem 415: 175–181.
Kim YS, Niazi JH, Gu MB (2009) Specific detection of oxytetracycline using DNA aptamer-immobilized interdigitated array electrode chip. Anal Chim Acta 634: 250–254.
Kim CH, Lee LP, Min JR, Lim MW, Jeong SH (2014) An indirect competitive assay-based aptasensor for detection of oxytetracycline in milk. Biosens Bioelectron 51:426–430.
Kouassi GK, Wang P, Sreevatan S, Irudayaraj J (2007) Aptamer-mediated magnetic and gold-coated magnetic nanoparticles as detection assay for prion protein assessment. Biotechnol Prog 23: 1239–1244
Cheng GF, Huang CH, Jie Z,Tan XL, He PG, Fang YZ (2009) A novel electrochemical biosensor for deoxyribonucleic acid detection based on magnetite nanoparticles. Chinese J Anal Chem 37: 169–173.
Wu SJ, Duan N, Wang ZP, Wang HX (2011) Aptamer-functionalized magnetic nanoparticle-based bioassay for the detection of ochratoxin a using upconversion nanoparticles as labels. Analyst 136: 2306–2314.
Yu Q, Yang H, Feng YM, Yang XL, Zhu YH (2012) Magnetic affinity enzyme-linked immunoassay based on recombinant 26 kDa glutathione-S-transferase for serological diagnosis of schistosomiasis japonica. Acta Trop 124: 199–202.
Bruno JG, Richarte AM, Phillips T (2014) Preliminary development of a DNA aptamer-magnetic bead capture electrochemiluminescence sandwich assay for brain natriuretic peptide. Microchem J 115: 32–38.
Barthelmebs L, Hayat A, Limiadi AW, Marty JL, Noguer T (2011) Electrochemical DNA aptamer-based biosensor for OTA detection, using superparamagnetic nanoparticles. Sensor. Actuat. B-Chem 156: 932–937.
Niazi JH, Lee SJ, Kim YS, Gu MB (2008) SsDNA aptamers that selectively bind oxytetracycline. Bioorgan Med Chem 16: 1254–1261.
Bulletin of the Ministry of Agriculture of the People’s Republic of China, No. 1025–20–2008.
Liu XJ, Jiang YY, Li MY, Wang H, Liu YJ, Lu LL, Sun YM, Lei HT (2013) Development of enzyme-linked aptamer assay for detection of kanamycin a. Chinese J Anal Chem 9: 1428–1433.
Duan N, Wu SJ, Zhu CQ, Ma XY, Wang ZP, Yu Y, Jiang Y (2012) Dual-color upconversion fluorescence and aptamer-functionalized magnetic nanoparticles-based bioassay for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Anal Chim Acta 723:1–6.
National Standard of the People’s Republic of China, GB/T 22990-2008.
National Standard of the People’s Republic of China, GB/T 18932.4-2002.
National Standard of the People’s Republic of China, GB/T 22995-2008.
National Standard of the People’s Republic of China, GB/T 22969-2008.
National Standard of the People’s Republic of China, GB/T 20764-2006.
National Standard of the People’s Republic of China, GB/T 22954-2008.
Wu SJ, Duan N, Ma XY, Xia Y, Wang HX, Wang ZP, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84: 6263–6270.
Zhao HM, Gao S, Liu M, Chang YY, Fan XF, Quan X (2013) Fluorescent assay for oxytetracycline based on a long-chain aptamer assembled onto reduced graphene oxide. Microchim Acta 180: 829–835.
Wongtangprasert T, Natakuathung W, Pimpitak U, Buakeaw A, Palaga T, Komolpis K, Khongchareonporn N (2014) Production of a monoclonal antibody against oxytetracycline and its application for oxytetracycline residue detection in shrimp. J Zhejiang Univ Sci B 15: 165–172.
Kim YS, Kim JH, Kim IA, Lee SJ, Jurng J, Gu MB (2010) A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens Bioelectron 26:1644–1649.
Fang CC, Wu SJ, Duan N, Dai SL, Wang ZP (2015) Highly sensitive aptasensor for oxytetracycline based on upconversion andmagnetic nanoparticles. Anal Methods doi:10.1039/C4AY03035D.
Bai XJ, Hou H, Zhang BL, Tang JL (2014) Label-free detection of kanamycin using aptamer-based cantilever array sensor. Biosens Bioelectron 56: 112–116
Zhu Y, Chandra P, Song KM, Ban C, Shim YB (2012) Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite. Biosens Bioelectron 36: 29–34.
Acknowledgments
This work is financially supported by the Science & Technology to guide project of Xinjiang Academy of Agriculture and Reclamation Science (62YYD201309).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Changbin Liu and Chunxia Lu contributed equally to this work.
Electronic supplementary material
ESM 1
(DOC 507 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Lu, C., Tang, Z. et al. Aptamer-functionalized magnetic nanoparticles for simultaneous fluorometric determination of oxytetracycline and kanamycin. Microchim Acta 182, 2567–2575 (2015). https://doi.org/10.1007/s00604-015-1628-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1628-z