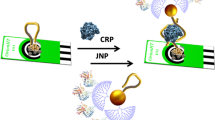
Abstract
We report on a sensitive aptamer-antibody interaction-based assay for cytochrome c (Cyt c) using electrochemical impedance. 4-Amino benzoic acid is used for the oriented immobilization of aminated aptamers onto multi-walled carbon nanotubes on the surface of a screen-printed electrode via electrochemical grafting. Impedance was measured in a solution containing the redox system ferro/ferricyanide. The change in interfacial charge transfer resistance (Rct) experienced by the redox marker was recorded to confirm the formation of a complex between aptamer and the target (Cyt c). A biotinylated antibody against cytochrome c was then used in a sandwich type of assay. The addition of streptavidin conjugated to gold nanoparticles and signal enhancement by treatment with silver led to a further increase in Rct. Under optimized conditions, a detection limit as low as 12 pM was obtained. Cross-reactivity against other serum proteins including fibrinogen, BSA and immunoglobulin G demonstrated improved selectivity.

Sensitive and selective assay for cytochrome c protein using aptamer linked to multi-walled carbon nanotube screen printed electrode via diazonium electrochemical grafting and specific biotinylated antibody to improve selectivity. Detection can be based on electrochemical impedance spectroscopy, or using a streptavidin-gold nanoparticle conjugate.






Similar content being viewed by others
References
Alleyne T, Joseph J, Sampson V (2001) Cytochrome-c detection: a diagnostic marker for myocardial infarction. Appl Biochem Biotechnol 90(2):97–105
Clark SL, Remcho VT (2002) Aptamers as analytical reagents. Electrophoresis 23(9):1335–1340
Radi A, Acero Sánchez JL, Baldrich E, O’Sullivan CK (2005) Reusable impedimetric aptasensor. Anal Chem 77(19):6320–6323
Biesecker G, Dihel L, Enney K, Bendele RA (1999) Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology 42(1–3):219–230
Hicke BJ, Marion C, Chang YF, Gould T, Lynott CK, Parma D, Schmidt PG, Warren S (2001) Tenascin-C aptamers are generated using tumor cells and purified protein. J Biol Chem 276(52):48644–48654
Cox JC, Ellington AD (2001) Automated selection of anti-protein aptamers. Bioorg Med Chem 9(10):2525–2531
Cai H, Lee TM-H, Hsing IM (2006) Label-free protein recognition using an aptamer-based impedance measurement assay. Sensors Actuators B Chem 114(1):433–437
Jayasena SD (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 45(9):1628–1650
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Tuerk C, Gold L (1990) Systemic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Tombelli S, Minunni M, Mascini M (2005) Analytical applications of aptamers. Biosens Bioelectron 20(12):2424–2434
Zhang L, Cui P, Zhang B, Gao F (2013) Aptamer-based turn-on detection of thrombin in biological fluids based on efficient phosphorescence energy transfer from Mn-doped ZnS quantum dots to carbon nanodots. Chem Eur J 19(28):9242–9250
Chang M, Kwon M, Kim S, Yunn NO, Kim D, Ryu SH, Lee JB (2014) Aptamer-based single-molecule imaging of insulin receptors in living cells. J Biomed Opt 19(5):051204
Loo AH, Bonanni A, Pumera M (2012) Impedimetric thrombin aptasensor based on chemically modified graphenes. Nanoscale 4(1):143–147
Ho MY, D’Souza N, Migliorato P (2012) Electrochemical aptamer-based sandwich assays for the detection of explosives. Anal Chem 84(10):4245–4247
Lei P, Tang H, Ding S, Ding X, Zhu D, Shen B, Chang Q, Yan Y (2015) Determination of the invA gene of Salmonella using surface plasmon resonance along with streptavidin aptamer amplification. Microchim Acta 182:289–296
Shen B, Li J, Cheng W, Yan Y, Tang R, Li Y, Ju H, Ding S (2015) Electrochemical aptasensor for highly sensitive determination of cocaine using a supramolecular aptamer and rolling circle amplification. Microchim Acta 182:361–367
McDonald JR (1987) Impedance spectroscopy. Wiley, New York
Bonanni A, Ambrosi A, Pumera M (2012) On oxygen-containing groups in chemically modified graphenes. Chem-Eur J 18(15):4541–4548
Bonanni A, Ambrosi A, Pumera M (2012) Nucleic acid functionalized graphene for biosensing. Chem-Eur J 18(6):1668–1673
Ocaña C, del Valle M (2014) A comparison of four protocols for the immobilization of an aptamer on graphite composite electrode. Microchim Acta 181:355–363
Bardea A, Patolsky F, Dagan A, Willner I (1999) Sensing and amplification of oligonucleotide-DNA interactions by means of impedance spectroscopy: a route to a Tay-Sachs sensor. Chem Comm 1:21–22
Loo AH, Bonanni A, Ambrosi A, Poh HL, Pumera M (2012) Impedimetric immunoglobulin G immunosensor based on chemically modified graphenes. Nanoscale 4(3):921–925
Moreno-Guzmán M, Ojeda I, Villalonga R, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM (2012) Ultrasensitive detection of adrenocorticotropin hormone (ACTH) using disposable phenylboronic-modified electrochemical immunosensors. Biosens Bioelectron 35(1):82–86
Zhang Y, Li Y, Wu W, Jiang Y, Hu B (2014) Chitosan coated on the layers’ glucose oxidase immobilized on cysteamine/Au electrode for use as glucose biosensor. Biosens Bioelectron 60:271–276
Bonanni A, Esplandiu MJ, del Valle M (2010) Impedimetric genosensing of DNA polymorphism correlated to cystic fibrosis: a comparison among different protocols and electrode surfaces. Biosens Bioelectron 26(4):1245–1251
Bonanni A, Pumera M, Miyahara Y (2010) Rapid, sensitive, and label-free impedimetric detection of a single-nucleotide polymorphism correlated to kidney disease. Anal Chem 82(9):3772–3779
Blin F, Koutsoukos P, Klepetsianis P, Forsyth M (2007) The corrosion inhibition mechanism of new rare earth cinnamate compounds—electrochemical studies. Electrochim Acta 52(21):6212–6220
Liao Y-M, Feng Z-D, Chen Z-L (2007) In situ tracing the process of human enamel demineralization by electrochemical impedance spectroscopy (EIS). J Dent 35(5):425–430
Bonanni A, del Valle M (2010) Use of nanomaterials for impedimetric DNA sensors: a review. Anal Chim Acta 678(1):7–17
Deng C, Chen J, Nie Z, Wang M, Chu X, Chen X, Xiao X, Lei C, Yao S (2008) Impedimetric aptasensor with femtomolar sensitivity based on the enlargement of surface-charged gold nanoparticles. Anal Chem 81(2):739–745
Zheng J, Feng W, Lin L, Zhang F, Cheng G, He P, Fang Y (2007) A new amplification strategy for ultrasensitive electrochemical aptasensor with network-like thiocyanuric acid/gold nanoparticles. Biosens Bioelectron 23(3):341–347
Bonanni A, Esplandiu MJ, del Valle M (2008) Signal amplification for impedimetric genosensing using gold-streptavidin nanoparticles. Electrochim Acta 53(11):4022–4029
Bonanni A, Esplandiu MJ, Pividori MI, Alegret S, del Valle M (2006) Impedimetric genosensors for the detection of DNA hybridization. Anal Bioanal Chem 385(7):1195–1201
Cai H, Wang YQ, He PG, Fang YH (2002) Electrochemical detection of DNA hybridization based on silver-enhanced gold nanoparticle label. Anal Chim Acta 469(2):165–172
Hanaee H, Ghourchian H, Ziaee AA (2007) Nanoparticle-based electrochemical detection of hepatitis B virus using stripping chronopotentiometry. Anal Biochem 370(2):195–200
Loo FC, Ng SP, Wu C-ML, Kong SK (2014) An aptasensor using DNA aptamer and white light common-path SPR spectral interferometry to detect cytochrome-c for anti-cancer drug screening. Sensors Actuators B: Chem 198:416–423
Lau IPM, Ngan EKS, Loo JFC, Suen YK, Ho HP, Kong SK (2010) Aptamer-based bio-barcode assay for the detection of cytochrome-c released from apoptotic cells. Biochem Biophys Res Commun 395:560–564
Liu JM, Yan XP (2011) Ultrasensitive, selective and simultaneous detection of cytochrome c and insulin based on immunoassay and aptamer-based bioassay in combination with Au/Ag nanoparticle tagging and ICP-MS detection. J Anal At Spectrom 26:1191–1197
Ocaña C, Arcay E, Del Valle M (2014) Label-free impedimetric aptasensor based on epoxy-graphite electrode for the recognition of cytochrome c. Sensors Actuators B Chem 191:860–865
Pandiaraj M, Benjamin AR, Madasamy T, Vairamani K, Arya A, Sethy NK (2014) A cost-effective volume miniaturized and microcontroller based cytochrome c assay. Sensors Actuators A Phys 220:290–297
Pandiaraj M, Madasamy T, Gollavilli PN, Balamurugan M, Kotamraju S, Rao (2013) Nanomaterial-based electrochemical biosensors for cytochrome c using cytochrome c reductase. Bioelectrochemistry 91:1–7
Sakaida I, Kimura T, Yamasaki T, Fukumoto Y, Watanabe K, Aoyama M, Okita K (2005) Cytochrome c is a possible new marker for fulminant hepatitis in humans. J Gastroenterol 40(2):179–185
Acknowledgments
This research was partly supported by the Research Executive Agency (REA) of the European Union under Grant Agreement number PITN-GA-2010-264772 (ITN CHEBANA), by the Ministry of Science and Innovation (MCINN, Madrid, Spain) through the project CTQ2013-41577-P and by the Catalonia program ICREA Academia. Cristina Ocaña thanks the support of Ministry of Science and Innovation (MICINN, Madrid, Spain) for the predoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 78 kb)
Rights and permissions
About this article
Cite this article
Ocaña, C., Lukic, S. & del Valle, M. Aptamer-antibody sandwich assay for cytochrome c employing an MWCNT platform and electrochemical impedance. Microchim Acta 182, 2045–2053 (2015). https://doi.org/10.1007/s00604-015-1540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1540-6