Abstract
Novel nanocomposites were prepared from graphene oxide (GO) and octahedral tin dioxide (SnO2) through a facile process that included synthesis of octahedral SnO2 and the reduction of GO with ascorbic acid. The morphology and structure of the nanocomposites were characterized by UV–vis spectroscopy, transmission electron microscopy, and Raman spectroscopy. The nanocomposites were placed on a glassy carbon electrode where they displayed excellent performance in terms of differential pulse voltammetric determination of dopamine (DA). This is attributed to (a) the synergetic interactions between reduced graphene oxide (r-GO) and octahedral SnO2, and (b) the presence of a large number of active sites on the nanocomposites surface. The sensor responds to DA in the concentration range of 0.08 to 30 μM, with a 6 nM detection limit if operated at 0.24 V (vs. Ag/AgCl). The modified electrode also widely suppresses the background current resulting from excess ascorbic acid and uric acids. The method was applied to the determination of DA in spiked human urine and gave satisfactory results, with recoveries in the range from 96.4 to 98.2 %.

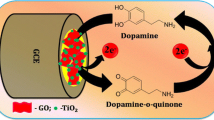
Green and facile synthesis of reduced graphene oxide-octahedral SnO2 (r-GO-SnO2) nanocomposites for the sensitive and selective electrochemical detection of dopamine.






Similar content being viewed by others
References
Bianco A, Cheng HM, Enoki T, Gogotsi Y, Hurt RH (2013) All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon 65:1–6
Hu Y, Li X, Geng D, Cai M, Li R, Sun X (2013) Influence of paper thickness on the electrochemical performances of graphene papers as an anode for lithium ion batteries. Electrochim Acta 91:227–233
Chakrabarti MH, Low CTJ, Brandon NP, Yufit V, Hashim MA (2013) Progress in the electrochemical modification of graphene-based materials and their applications. Electrochim Acta 107:424–440
Huang KJ, Liu YJ, Wang HB, Gan T, Liu YM, Wang LL (2014) Signal amplification for electrochemical DNA biosensor based on two-dimensional graphene analogue tungsten sulfide-grapheme composites and gold nanoparticles. Sensors Actuators B 191:828–836
Huang KJ, Liu YJ, Cao JT, Wang HB (2014) An aptamer electrochemical assay for sensitive detection of immunoglobulin E based on tungsten disulfide-graphene composites and gold nanoparticles. RSC Adv 4(69):36742–36748
Huang KJ, Liu YJ, Wang HB, Wang YY (2014) A sensitive electrochemical DNA biosensor based on silver nanoparticles-polydopamine@graphene composite. Electrochim Acta 118:130–137
Huang KJ, Wang L, Li J, Yu M (2013) Electrochemical sensing of catechol using a glassy carbon electrode modified with a composite made from silver nanoparticles, polydopamine, and grapheme. Microchim Acta 180:751–757
Wang C, Zhou Y, Ge MY, Xu XB, Zhang ZL, Jiang JZ (2010) Large-scale synthesis of SnO2 nanosheets with high lithium storage capacity. J Am Chem Soc 132(1):46–47
Jiang LY, Wu XL, Guo YG, Wan LJ (2009) SnO2-based hierarchical nanomicrostructures: facile synthesis and their applications in gas sensors and lithium-ion batteries. J Phys Chem C 113(32):14213–14219
Selvan RK, Perelshtein I, Perkas N, Gedanken A (2008) Synthesis of hexagonal-shaped SnO2 nanocrystals and SnO2@C nanocomposites for electrochemical redox supercapacitors. J Phys Chem C 112:1825–1830
Wang YF, Li JW, Hou YF, Yu XY, Su CY, Kuang DB (2010) Hierarchical tin oxide octahedra for highly efficient dye-sensitized solar cells. Chem Eur J 16(29):8620–8625
Kong FY, Li WW, Wang JY, Wang W (2015) UV-assisted photocatalytic synthesis of highly dispersed Ag nanoparticles supported on DNA decorated graphene for quantitative iodide analysis. Biosensor Bioelectron 69:206–212
Kong FY, Li XR, Zhao WW, Xu JJ, Chen HY (2012) Graphene oxide-thionine-Au nanostructure composites: preparation and applications in non-enzymatic glucose sensing. Electrochem Commun 14:59–62
Lee JH, Ribeiro C, Giraldi TR, Longo E, Leite ER, Varela JA (2004) Photoluminescence in quantum-confined SnO2 nanocrystals: evidence of free exciton decay. Appl Phys Lett 84(10):1745–1747
Baek S, Yu SH, Park SK, Pucci A, Marichy C, Lee DC, Sung YE, Piao Y, Pinna N (2011) A one-pot microwave-assisted non-aqueous sol–gel approach to metal oxide/graphene nanocomposites for li-ion batteries. RSC Adv 1(9):1687–1690
Lian P, Zhu X, Liang S, Li Z, Yang W, Wang H (2010) Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim Acta 55(12):3909–3914
Li YM, Lv XJ, Lu J, Li JH (2010) Preparation of SnO2-nanocrystal/graphene-nanosheets composites and their lithium storage ability. J Phys Chem C 114(49):21770–21774
Wang HL, Robinson JT, Li XL, Dai HJ (2009) Solvothermal reduction of chemically exfoliated graphene sheets. J Am Chem Soc 131(29):9910–9911
Bourlinos AB, Gournis D, Petridis D, Szabó T, Szeri A, Dékány I (2003) Graphite oxide: chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 19(15):6050–6055
Xu CH, Sun J, Gao L (2012) Controllable synthesis of monodisperse ultrathin SnO2 nanorods onnitrogen-doped grapheme and its ultrahigh lithium storage properties. Nanoscale 4(17):5425–5430
Zhang M, Lei D, Du ZF, Yin XM, Chen LB, Li QH, Wang YG, Wang TH (2011) Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J Mater Chem 21(6):1673–1676
Zhang JL, Yang HJ, Shen GX, Cheng P, Guo SW (2010) Reduction of graphene oxide via L-ascorbic acid. Chem Commun 46(7):1112–1114
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182(1–2):1–41
Breczkoa J, Plonska-Brzezinskaa ME, Echegoyenb L (2012) Electrochemical oxidation and determination of dopamine in the presence of uric and ascorbic acids using a carbon nano-onion and poly(diallyldimethylammonium chloride) composite. Electrochim Acta 72:61–67
Yang AK, Xue Y, Zhang Y, Zhang XF, Zhao H, Li XG, He YJ, Yuan ZB (2013) A simple one-pot synthesis of graphene nanosheet/SnO2 nanoparticle hybrid nanocomposites and their application for selective and sensitive electrochemical detection of dopamine. J Mater Chem B 1(13):1804–1811
Sun W, Wang XZ, Wang YH, Ju XM, Xu L, Li GG, Sun ZF (2013) Application of graphene-SnO2 nanocomposite modified electrode for the sensitive electrochemical detection of dopamine. Electrochim Acta 87:317–322
Zhang FY, Li YJ, Gu YE, Wang ZH, Wang CM (2011) One-pot solvothermal synthesis of a Cu2O/Graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173(1–2):103–109
Li SJ, Deng DH, Shi Q, Liu SR (2012) Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim Acta 177(3–4):325–331
Dumitrescu I, Edgeworth JP, Unwin PR, Macpherson JV (2009) Ultrathin carbon nanotube mat electrodes for enhanced amperometric detection. Adv Mater 21(30):3105–3109
Tu XM, Xie QJ, Jiang SY, Yao SZ (2007) Electrochemical quartz crystal impedance study on the overoxidation of polypyrrole carbon nanotubes composite film for amperometric detection of dopamine. Biosens Bioelectron 22(12):2819–2826
Moreno M, Arribasa AS, Bermejo E, Chicharro M (2010) Selective detection of dopamine in the presence of ascorbic acid using carbon nanotube modified screen-printed electrodes. Talanta 80(5):2149–2156
Adekunle AS, Agboola BO, Kenneth JP, Ozoemena KI (2010) Electrocatalytic detection of dopamine at single-walled carbon nanotubes-iron (III) oxide nanoparticles platform. Sensors Actuators B 148(1):93–102
Deng CY, Chen JH, Wang MD, Xiao CH, Nie Z, Yao SZ (2009) A novel and simple strategy for selective and sensitive determination of dopamine based on the boron-doped carbon nanotubes modified electrode. Biosens Bioelectron 24(7):2091–2094
Liu S, Yan J, He GW, Zhong DD, Chen JX, Shi LY, Zhou XM, Jiang HJ (2012) Layer-by-layer assembled multilayer films of reduced graphene oxide/gold nanoparticles for the electrochemical detection of dopamine. J Electroanal Chem 672:40–44
Kalimuthu P, John SA (2010) Simultaneous determination of ascorbic acid, dopamine, uric acid and xanthine using a nanostructured polymer film modified electrode. Talanta 80:1686–1691
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21305122), the Natural Science Foundation of Jiangsu Province (BK20131218), the Opening Foundation of the State Key Laboratory of Analytical Chemistry for Life Science of Nanjing University (SKLACLS1312), and the Industry-University-Research Cooperative Innovation Foundation of Jiangsu Province (BY2014108-19, BY2014108-08).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 851 kb)
Rights and permissions
About this article
Cite this article
Ma, HF., Chen, TT., Luo, Y. et al. Electrochemical determination of dopamine using octahedral SnO2 nanocrystals bound to reduced graphene oxide nanosheets. Microchim Acta 182, 2001–2007 (2015). https://doi.org/10.1007/s00604-015-1521-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1521-9