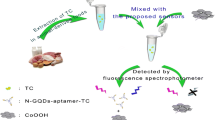
Abstract
We have developed an aptamer-based assay for the food toxin ricin. It is based on a competitive quenching strategy along with time-resolved fluorescence detection. The assay involves the following steps: (a) The aptamer is immobilized on the europium-doped KGdF4 nanoparticles (NPs); (b) these NPs are added to the sample where ricin binds to the aptamer; (c) graphene oxide (GO) is added and competitively binds to the aptamer on the NPs that are not blocked by ricin; this causes the quenching of the fluorescence of the NPs; (d) fluorescence is detected at 593 nm in a microplate reader in the time-resolved mode at an excitation wavelength of 273 nm, a delay time of 100 μs, and a gating time of 1 s. Under optimal conditions, the calibration plot is linearly related to the concentration of ricin in the 50 pg·mL−1 to 50 ng·mL−1 range (R2 = 0.9975), and the limit of detection is 8 pg·mL−1. The method was compared to a standard ELISA, and correlation was excellent. The assay presented here provides a sensitive, dependable and convenient platform that is expected to have promising applications for the homogeneous assay of various other target analytes.

A homogeneous time-resolved fluorescence assay was developed for the determination of ricin in portable water by FRET between KGdF4:Eu3+ nanoparticles-tagged aptamer and graphene oxide, with a good linearity and the limit of detection of 0.8 × 10−11 g mL−1.





Similar content being viewed by others
References
Olsnes S, Pihl A (1973) Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 12(16):3121–3126. doi:10.1021/bi00740a028
Olsnes S, Refsnes K, Pihl A (1974) Mechanism of action of the toxic lectins abrin and ricin. Nature 249(458):627–631. doi:10.1038/249627a0
Lord JM, Roberts LM, Robertus JD (1994) Ricin - structure, mode of action, and some current applications. FASEB J 8(2):201–208
Jackson LS, Zhang Z, Tolleson WH (2010) Thermal stability of ricin in orange and apple juices. J Food Sci 75(4):T65–T71. doi:10.1111/j.1750-3841.2010.01570.x
Brandon DL (2011) Detection of ricin contamination in ground beef by electrochemiluminescence immunosorbent assay. Toxins 3(4):398–408. doi:10.3390/toxins3040398
Shyu HF, Chiao DJ, Liu HW, Tang SS (2002) Monoclonal antibody-based enzyme immunoassay for detection of ricin. Hybridomas Hybridomas 21(1):69–73. doi:10.1089/15368590252917665
Anderson GP, Glaven RH, Algar WR, Susumu K, Stewart MH, Medintz IL, Goldman ER (2013) Single domain antibody-quantum dot conjugates for ricin detection by both fluoroimmunoassay and surface plasmon resonance. Anal Chim Acta 786:132–138. doi:10.1016/j.aca.2013.05.010
Suresh S, Gupta AK, Rao VK, Kumar O, Vijayaraghavan R (2010) Amperometric immunosensor for ricin by using on graphite and carbon nanotube paste electrodes. Talanta 81(1–2):703–708. doi:10.1016/j.talanta.2010.01.007
Garber EAE, O’Brien TW (2008) Detection of ricin in food using electrochemiluminescence-based technology. J AOAC Int 91(2):376–382
Shyu RH, Shyu HF, Liu HW, Tang SS (2002) Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 40(3):255–258. doi:10.1016/S0041-0101(01)00193-3
Wu JJ, Zhu YY, Xue F, Mei ZL, Yao L, Wang X, Zheng L, Liu J, Liu GD, Peng CF, Chen W (2014) Recent trends in SELEX technique and its application to food safety monitoring. Microchim Acta 181(5–6):479–491. doi:10.1007/s00604-013-1156-7
Valdes MG, Gonzalez ACV, Calzon JAG, Diaz-Garcia ME (2009) Analytical nanotechnology for food analysis. Microchim Acta 166(1–2):1–19. doi:10.1007/s00604-009-0165-z
Lamont EA, He LL, Warriner K, Labuza TP, Sreevatsan S (2011) A single DNA aptamer functions as a biosensor for ricin. Analyst 136(19):3884–3895. doi:10.1039/C1an15352h
Zhang HQ, Li F, Dever B, Li XF, Le XC (2013) DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem Rev 113(4):2812–2841. doi:10.1021/Cr300340p
Yan JL, Estevez MC, Smith JE, Wang KM, He XX, Wang L, Tan WH (2007) Dye-doped nanoparticles for bioanalysis. Nano Today 2(3):44–50. doi:10.1016/s1748-0132(07)70086-5
Ma Q, Su XG (2011) Recent advances and applications in QDs-based sensors. Analyst 136(23):4883–4893. doi:10.1039/c1an15741h
Tu DT, Liu LQ, Ju Q, Liu YS, Zhu HM, Li RF, Chen XY (2011) Time-resolved FRET biosensor based on amine-functionalized lanthanide-doped nayf4 nanocrystals. Angew Chem Int Ed 50(28):6306–6310. doi:10.1002/anie.201100303
Sapsford KE, Berti L, Medintz IL (2006) Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations. Angew Chem Int Ed 45(28):4562–4588. doi:10.1002/anie.200503873
Wang Y, Li Z, Wang J, Li J, Lin Y (2011) Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29(5):205–212. doi:10.1016/j.tibtech.2011.01.008
Perez-Lopez B, Merkoci A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179(1–2):1–16. doi:10.1007/s00604-012-0871-9
Pang S, Gao Y, Li Y, Liu SY, Su XG (2013) A novel sensing strategy for the detection of Staphylococcus aureus DNA by using a graphene oxide-based fluorescent probe. Analyst 138(9):2749–2754. doi:10.1039/C3an36642a
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181(5–6):647–653. doi:10.1007/s00604-014-1170-4
Tang JJ, Xie JW, Shao NS, Yan Y (2006) The DNA aptamers that specifically recognize ricin toxin are selected by two in vitro selection methods. Electrophoresis 27(7):1303–1311. doi:10.1002/elps.200500489
Ju Q, Tu DT, Liu YS, Li RF, Zhu HM, Chen JC, Chen Z, Huang MD, Chen XY (2012) Amine-functionalized lanthanide-doped KGdF4 nanocrystals as potential optical/magnetic multimodal bioprobes. J Am Chem Soc 134(2):1323–1330. doi:10.1021/Ja2102604
Huang YK, Chen XJ, Xia Y, Wu SJ, Duan N, Ma XY, Wang ZP (2014) Selection, identification and application of a DNA aptamer against Staphylococcus aureus enterotoxin A. Anal Methods-UK 6(3):690–697. doi:10.1039/C3ay41576g
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1
Eliseeva SV, Buenzli J-CG (2010) Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev 39(1):189–227. doi:10.1039/b905604c
Wang GF, Peng Q, Li YD (2011) Lanthanide-doped nanocrystals: synthesis, optical-magnetic properties, and applications. Accounts Chem Res 44(5):322–332. doi:10.1021/ar100129p
Appelblom H, Sipponen A, Valanne A, Soukka T, Lovgren T, Niemela P (2011) Antibody-free lanthanide-based fluorescent probe for determination of protein tyrosine kinase and phosphatase activities. Microchim Acta 172(1–2):25–29. doi:10.1007/s00604-010-0450-x
Du YP, Zhang YW, Sun LD, Yan CH (2009) Optically active uniform potassium and lithium rare earth fluoride nanocrystals derived from metal trifluroacetate precursors. Dalton Trans 40:8574–8581. doi:10.1039/B909145a
Shen JF, Hu YZ, Shi M, Lu X, Qin C, Li C, Ye MX (2009) Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem Mater 21(15):3514–3520. doi:10.1021/Cm901247t
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39(1):228–240. doi:10.1039/b917103g
Varghese N, Mogera U, Govindaraj A, Das A, Maiti PK, Sood AK, Rao CNR (2009) Binding of DNA nucleobases and nucleosides with graphene. ChemPhysChem 10(1):206–210. doi:10.1002/cphc.200800459
Feldmann C, Justel T, Ronda CR, Schmidt PJ (2003) Inorganic luminescent materials: 100 years of research and application. Adv Funct Mater 13(7):511–516. doi:10.1002/adfm.200301005
Acknowledgments
This work was partly supported by the National S&T Support Program of China (2012BAK08B01), S&T Supporting Project of Jiangsu Province (BE2011621), NCET-11-0663, and JUSRP51309A.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 420 kb)
Rights and permissions
About this article
Cite this article
Huang, Y., Chen, X., Wu, S. et al. Homogeneous time-resolved fluorescence assay for the detection of ricin using an aptamer immobilized on europium-doped KGdF4 nanoparticles and graphene oxide as a quencher. Microchim Acta 182, 1035–1043 (2015). https://doi.org/10.1007/s00604-014-1422-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1422-3