Abstract
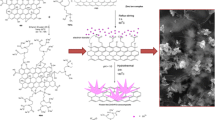
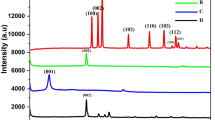
We describe a novel procedure for the synthesis of nitrogen-doped reduced graphene oxide (N-rGO). It is based on the thermal reduction of GO (dispersed in water) with sodium diethyldithiocarbamate that acts as both the reducing agent and the source for nitrogen. The surface morphology of the N-rGO is characterized using high resolution transmission electron microscopy. X-ray photoelectron spectroscopy was carried out to study the composition of their surface, and Raman spectroscopy was performed to study the level of doping with nitrogen and the structural order. The N-rGO was deposited on a glassy carbon electrode (GCE), and the resulting electrode utilized as a sensing platform for 4-nitrophenol (4-NP). The modified GCE exhibits a well-defined oxidation peak current that is about ten times larger when compared to that of a bare GCE. The electron transfer number, proton transfer number and electron transfer rate constant (ks 1.046 s−1) were determined. At optimized conditions, the oxidation peak current is linearly related to the concentration of 4-NP in the 20–500 nM range, with a correlation coefficient of 0.9917. The detection limit (at an SNR of 3) is 7 nM. The method was successfully applied to the analysis of waters spiked with 4-NP. Recoveries range from 97.8 to 102.6 %, and no interferences are found for common inorganic cations and anions.

ᅟ








Similar content being viewed by others
References
Lacorte S, Barceto D (1994) Rapid degradation of fenitrothion in estuarine waters. Environ Sci Technol 28:1159–1163
Francis PC, Grothe DW, Scheuring JC (1986) Chronic toxicity of 4-nitrophenol to daphnia magna straus under statis-renewal and flow-through conditions. Bull Environ Contam Toxicol 36:730–737
ATSDR, (1992) Toxicological profile for nitrophenols, agency for toxic substances and disease registry, Atlanta
Herterich R (1991) Gas chromatographic determination of nitrophenols in atmospheric liquid water and airborne particulates. J Chromatogr A 549:313–324
Daz TG, Cabanillas AG, Dez NM, Vzquez PP, Lpez FS (2000) Rapid and sensitive determination of 4-nitrophenol, 3-methyl-4-nitrophenol, 4,6-dinitro-o-cresol, parathion-methyl, fenitrothion, and parathion-ethyl by liquid chromatography with electrochemical detection. J Agric Food Chem 48:4508–4513
Giribabu K, Suresh R, Manigandan R, Munusamy S, Praveen Kumar S, Muthamizh S, Narayanan V (2013) Nanomolar determination of 4-nitrophenol based on a poly (methylene blue)-modified glassy carbon electrode. Analyst 138:5811–5818
Yin H, Zhou Y, Ai S, Liu X, Zhu L, Lu L (2010) Electrochemical oxidative determination of 4-nitrophenol based on a glassy carbon electrode modified with a hydroxyapatite nanopowder. Microchim Acta 169:87–92
Luo L, Zou X, Ding Y, Wu Q (2008) Derivative voltammetric direct simultaneous determination of nitrophenol isomers at a carbon nanotube modified electrode. Sensors Actuators B 135:61–65
Arvinte A, Mahosenaho M, Pinteala M, Sesay AM, Virtanen V (2011) Electrochemical oxidation of p-nitrophenol using graphene-modified electrodes, and A comparison to the performance of MWNT-based electrodes, Microchim. Acta 174:337–343
Hu S, Xu C, Wang G, Cui D (2001) Voltammetric determination of 4-nitrophenol at a sodium montmorillonite-anthraquinone chemically modified glassy carbon electrode. Talanta 54:115–123
Bebeselea A, Manea F, Burtica G, Nagy L, Nagy G (2010) The electrochemical determination of phenolic derivates using multiple pulsed amperometry with graphite based electrodes. Talanta 80:1068–1072
Xu X, Liu Z, Zhang X, Duan S, Xu S, Zhou C (2011) β-Cyclodextrin functionalized mesoporous silica for electrochemical selective sensor: Simultaneous determination of nitrophenol isomers Electrochim. Acta 58:142–149
Xu Y, Wang Y, Ding Y, Luo L, Liu X, Zhang Y (2011) Electrochemical simultaneous determination of nitrophenol isomers at nano-gold modified glassy carbon electrode. J Appl Electrochem 41:687–697
Xu G, Yang L, Zhong M, Li C, Lu X, Kan X (2013) Selective recognition and electrochemical detection of p-nitrophenol based on a macroporous imprinted polymer containing gold nanoparticles, Microchim. Acta 180:1461–1469
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS (2012) Functionalization of graphene: covalent and Non-covalent approaches, derivatives and applications. Chem Rev 112:6156–6214
Gan T, Hu S (2011) Electrochemical sensors based on graphene materials. Microchim Acta 175:1–19
Deng D, Pan X, Yu L, Cui Y, Jiang Y, Qi J, Li WX, Fu Q, Ma X, Xue Q, Sun G, Bao X (2011) Toward N-doped graphene via solvothermal synthesis. Chem Mater 23:1188–1193
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Hu C, Liu Y, Yang Y, Cui J, Huang Z, Wang Y, Yang L, Wang H, Xiao Y, Rong J (2013) One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J Mater Chem B 1:39–42
Matter PH, Zhang L, Ozkan US (2006) The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J Catal 239:83–96
Jansen RJJ, Vanbekkum H (1995) XPS of nitrogen-containing functional groups on activated carbon. Carbon 33:1021–1027
Nakayama Y, Soeda F, Ishitani A (1990) XPS study of the carbon fiber matrix interface. Carbon 28:21–26
Malitesta C, Losito I, Sabbatini L, Zambonin PG (1995) New findings on polypyrrole chemical structure by XPS coupled to chemical derivatization labelling. J Electron Spectrosc Relat Phenom 76:629–634
Niwa Y, Kobayash H, Tsuchiya T (1974) X‐ray photoelectron spectroscopy of tetraphenylporphin and phthalocyanine. J Chem Phys 60:799–807
Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS (2009) Raman spectroscopy in graphene. Phys Rep 473:51–87
Yasuda S, Yu L, Kim J, Murakoshi K (2013) Selective nitrogen doping in graphene for oxygen reduction reactions. Chem Commun 49:9627–9629
Brownson DAC, Munro LJ, Kampouris DK, Banks CE (2011) Electrochemistry of graphene: not such a beneficial electrode material? RSC Adv 1:978–988
Harata K (1977) The Structure of the Cyclodextrin Complex. V. Crystal Structures of α-Cyclodextrin Complexes with p-Nitrophenol and p-Hydroxybenzoic Acid. Bull Chem Soc Jpn 50:1416–1424
Hirata M, Gotou T, Horiuchi S, Fujiwara M, Ohba M (2004) Thin-film particles of graphite oxide 1: high-yield synthesis and flexibility of the particles. Carbon 42:2929–2937
Konkena B, Vasudevan S (2012) Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements. J Phys Chem Lett 3:867–872
Zhou C, Liu Z, Dong Y, Li D (2009) Electrochemical behavior of o-nitrophenol at hexagonal mesoporous silica modified carbon paste electrodes. Electroanalysis 21:853–858
Li J, Kuang D, Feng Y, Zhang F, Xu Z, Liu M (2012) A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J Hazard Mater 201–202:250–259
Liu Z, Du J, Qiu C, Huang L, Ma H, Shen D, Ding Y (2009) Electrochemical sensor for detection of p-nitrophenol based on nanoporous gold. Electrochem Commun 11:1365–1368
Niaz A, Fischer J, Barek J, Yosypchuk B, Bhanger SMI (2009) Voltammetric determination of 4-nitrophenol using a novel type of silver amalgam paste electrode. Electroanalysis 21:1786–1791
Sun W, Yang MX, Jiang Q, Jiao K (2008) Direct electrocatalytic reduction of p-nitrophenol at room temperature ionic liquid modified electrode. Chin Chem Lett 19:1156–1158
Acknowledgements
One of the authors (K. Giribabu) wish to thank Department of Science and Technology (DST), Government of India, for the financial assistance in the form of INSPIRE fellowship (Inspire Fellow no :10226) under the AORC scheme. Authors thank National Center for Nanoscience and Nanotechnology (NCNSNT), University of Madras, for recording HR-TEM and XPS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 665 kb)
Rights and permissions
About this article
Cite this article
Giribabu, K., Suresh, R., Manigandan, R. et al. Preparation of nitrogen-doped reduced graphene oxide and its use in a glassy carbon electrode for sensing 4-nitrophenol at nanomolar levels. Microchim Acta 181, 1863–1870 (2014). https://doi.org/10.1007/s00604-014-1251-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1251-4