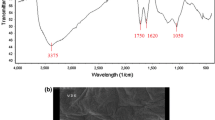
Abstract
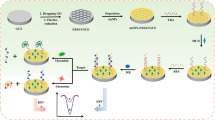
We have developed a “turn on” model of an electrochemiluminescence (ECL) based assay for lead ions. It is based on the formation of a G-quadruplex from an aptamer labeled with quantum dots (QDs) and placed on an electrode modified with of graphene and gold nanoparticles (AuNPs). A hairpin capture probe was labeled with a thiol group at the 5′-end and with an amino group at the 3′-end. It was then self-assembled on the electrode modified with graphene and AuNPs. In the absence of Pb(II), the amino tag on one end of the hairpin probe is close to the surface of the electrode and therefore unable to interact with the QDs because of steric hindrance. The ECL signal is quite weak in this case. If, however, Pb(II) is added, the stem-loop of the aptamer unfolds to form a G-quadruplex. The amino group at the 3′-end will become exposed and can covalently link to a carboxy group on the surface of the CdTe QDs. This leads to strong ECL. Its intensity increases (“turns on”) with the concentration of Pb(II). Such a “turn-on” method does not suffer from the drawbacks of “turn-off” methods. ECL intensity is linearly related to the concentration of Pb(II) in the 10 p mol·L−1 to 1 n mol·L−1 range, with a 3.8 p mol·L−1 detection limit. The sensor exhibits very low detection limits, good selectivity, satisfying stability, and good repeatability.

A “turn on” model of ECL method was developed based on G-quadruplex of Graphene/AuNPs of aptamer probe by using quantum dots as label. ECL intensity is increased with the increase of Pb2+ concentration. The responsive ECL intensity was linearly related to the Pb2+ concentration in the range of 1.0 × 10−11 ~ 1.0 × 10−9 mol·L−1, with a detection limit of 3.82 × 10−12 mol·L−1.







Similar content being viewed by others
References
Zapata F, Caballero A, Espinosa A, Trraga A, Molina P (2008) Triple channel sensing of Pb(II) ions by a simple multiresponsive ferrocene receptor having a 1-deazapurine backbone. Org Lett 10:41–44
Yang HJ, Jiang SJ (1995) Hydride generation inductively coupled plasma mass spectrometric detection of lead compounds separated by liquid chromatography. J Anal At Spectrom 10:963–967
Lemos VA, Guardia MDL, Ferreira SLC (2002) An on-line system for preconcentration and determination of lead in wine samples by FAAS. Talanta 58:475–480
Webb E, Amarasiriwardena D, Tauch S, Green EF, Jones J, Goodman AH (2005) Inductively coupled plasma-mass (ICP-MS) and atomic emission spectrometry (ICP-AES): versatile analytical techniques to identify the archived elemental information in human teeth. Microchem J 81:201–208
Lee YF, Huang CC (2011) Colorimetric assay of lead ions in biological samples using a nanogold-based membrane. Appl Mater Interfaces 3:2747–2754
Li T, Dong SJ, Wang EK (2010) A lead(II)-driven DNA molecular device for turn-on fluorescence detection of lead(ii) ion with high selectivity and sensitivity. J Am Chem Soc 132:13156–13157
Lin ZZ, Chen Y, Li XH, Fang WH (2011) Pb2+ induced DNA conformational switch from hairpin to G-quadruplex: electrochemical detection of Pb2+. Analyst 136:2367–2372
Li F, Feng Y, Zhao C, Tang B (2011) Crystal violet as a G-quadruplex-selective probe for sensitive amperometric sensing of lead. Chem Commun 47:11909–11911
Evtugyn G, Porfireva A, Ryabova M, Hianik T (2008) Aptasensor for thrombin based on carbon nanotubes-methylene blue composites. Electroanalysis 20:2310–2316
Sotiropoulou S, Chaniotakis NA (2003) Carbon nanotube array-based biosensor. Anal Bioanal Chem 375:103–105
Li XS, Cai WW, An JH, Kim SY, Nah J, Yang DX, Piner R, Velamakanni A, Jung I, Tutuc E, Banerjee SK, Colombo L, Ruoff RS (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324:1312–1314
Elias DC, Nair RR, Mohiuddin TMG, Morozov SV, Blake P, Halsall MP, Ferrari AC, Boukhvalov DW, Katsnelson MI, Geim AK, Novoselov KS (2009) Control of graphene’s properties by reversible hydrogenation: evidence for graphane. Science 323:610–613
Pumera M (2013) Electrochemistry of graphene, graphene oxide and other graphenoids: Review. Electrochem Commun 36:14–18
Xu C, Wang X, Zhu JW (2008) Graphene-metal particle nanocomposites. J Phys Chem C 112:19841–19845
Zhong ZY, Wu W, Wang D, Wang D, Shan JL, Qing Y, Zhang ZM (2010) Nanogold-enwrapped graphene nanocomposites as trace labels for sensitivity enhancement of electrochemical immunosensors in clinical immunoassays: carcinoembryonic antigen as a model. Biosens Bioelectron 25:2379–2383
Pérez-López B, Merkoçi A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16
Deng SY, Lei JP, Cheng LX, Zhang YY, Ju HX (2011) Amplified electrochemiluminescence of quantum dots by electrochemically reduced graphene oxide for nanobiosensing of acetylcholine. Biosens Bioelectron 26:4552–4558
Zhang LH, Zou XQ, Ying EB, Dong SJ (2008) Quantum dot electrochemiluminescence in aqueous solution at lower potential and its sensing application. J Phys Chem C 112:4451–4554
Zhang L, Shang L, Dong S (2008) Sensitive and selective determination of Cu by electrochmiluminescence of CdTe quantum dots. Electrochem Commun 10:1452–1454
Hai H, Yang F, Li JP (2013) Electrochemiluminescence sensor using quantum dots based on a G-quadruplex aptamer for the detection of Pb2+. RSC Adv 3:13144–13148
Yuan JP, Gaponik NKL, Eychmüller A (2012) Application of polymer quantum dot-enzyme hybrids in the biosensor development and test paper fabrication. Anal Chem 84:5047–5052
Huang H, Zhu JJ (2013) The electrochemical applications of quantum dots. Analyst 138:5855–5865
Li L-L, Liu K-P, Yang G-H, Wang C-M, Zhang J-R, Zhu J-J (2011) Fabrication of graphene-quantum dots composites for sensitive electrogenerated chemiluminescence immunosensing. Adv Funct Mater 21:869–878
Li L-L, Ji J, Fei R, Wang C-Z, Lu Q, Zhang J-R, Jiang L-P, Zhu J-J (2012) A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv Funct Mater 22:2971–2979
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun ZZ, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Han HY, Sheng ZH, Liang JG (2007) Electrogenerated chemiluminescence from thiol-capped CdTe quantum dots and its sensing application in aqueous solution. Anal Chim Acta 596:73–78
Li JP, Li SH, Wei XP, Tao HL, Pan HC (2012) Molecularly imprinted electrochemical luminescence sensor based on signal amplification for selective determination of trace Gibberellin A3. Anal Chem 84:9951–9955
Shan CS, Yang HF, Han DX, Zhang QX, Ivaska A, Niu L (2010) Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens Bioelectron 25:1070–1074
Cai H, Xu Y, He PG, Fang YZ (2004) Advances of the deoxyribonucleic acid immobilization on electrode surface for the electrochemical deoxyribonucleic acid biosensor designing. Chin J Anal Chem 32:815–820
Yu WW, Qu LH, Guo WZ, Peng XG (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Zhang XB, Wang ZD, Xing H, Xiang Y, Lu Y (2010) Catalytic and molecular beacons for amplified detection of metal ions and organic molecules with high sensitivity. Anal Chem 82:5005–5011
Radi AE, O’Sullivan CK (2006) Aptamer conformational switch as sensitive electrochemical biosensor for potassium ion recognition. Chem Commun 32:3432–3434
Wang HY, Chen QF, Tan Z, Yin XX, Wang L (2012) Electrochemiluminescence of CdTe quantum dots capped with glutathione and thioglycolic acid and its sensing of Pb2+. Electrochim Acta 72:28–31
Cheng LX, Liu X, Lei JP, Ju HX (2010) Low-potential electrochemiluminescent sensing based on surface unpassivation of CdTe quantum dots and competition of analyte cation to stabilizer. Anal Chem 82:3359–3364
Li T, Wang EK, Dong SJ (2009) Potassium-lead-switched g-quadruplexes: a new class of DNA logic gates. J Am Chem Soc 131:15082–15083
Lin ZZ, Li XH, Kraatz HB (2011) Impedimetric immobilized DNA-Based sensor for simultaneous detection of Pb2+, Ag+, and Hg2+. Anal Chem 83:6896–6901
Zhou XH, Kong DM, Shen HX (2010) Ag+ and cysteine quantitation based on G-quadruplex-hemin DNAzymes disruption by Ag+. Anal Chem 82:789–793
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ellington AD, Szostak JW (1992) Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 355:850–852
Jayasena SD (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 45:1628–1650
Hermann T, Patel DJ (2000) Adaptive recognition by nucleic acid aptamers. Science 287:820–825
Chen X, Guan HL, He ZK, Zhou XD, Hu JM (2012) A sensitive and selective label-free DNAzyme-based sensor for lead ions by using a conjugated polymer. Anal Methods 4:1619–1622
Fu XB, Qu F, Li NB, Luo HQ (2012) A label-free thrombin binding aptamer as a probe for highly sensitive and selective detection of lead(II) ions by a resonance Rayleigh scattering method. Analyst 137:1097–1099
Guo LQ, Nie DD, Qiu CY, Zheng QS, Wu HY, Ye PR, Hao YL, Fu FF, Chen GN (2012) A G-quadruplex based label-free fluorescent biosensor for lead ion. Biosens Bioelectron 35:123–127
Zhu X, Lin ZY, Chen LF, Qiu B, Chen GN (2009) A sensitive and specific electrochemiluminescent sensor for lead based on DNAzyme. Chem Commun 40:6050–6052
Zhao XH, Kong RM, Zhang XB, Meng HM, Liu WN, Tan WH, Shen GL, Yu RQ (2011) Graphene–DNAzyme based biosensor for amplified fluorescence “Turn-On” detection of Pb2+ with a high selectivity. Anal Chem 83:5062–5066
Zhao Y, Zhang Q, Wang W, Jin Y (2013) Input-dependent induction of G-quadruplex formation for detection of lead(II) by fluorescent ion logic gate. Biosens Bioelectron 43:231–236
Li T, Wang EK, Dong SJ (2010) Lead(II)-Induced allosteric G-Quadruplex DNAzyme as a colorimetric and chemiluminescence sensor for highly sensitive and selective Pb2+ detection. Anal Chem 82:1515–1520
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 21165007, 21375031) and the Natural Science Foundation of Guangxi Province of China (No. 2012GXNSFAA053032).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 45.7 kb)
Rights and permissions
About this article
Cite this article
Hai, H., Yang, F. & Li, J. Highly sensitive electrochemiluminescence “turn-on” aptamer sensor for lead(II) ion based on the formation of a G-quadruplex on a graphene and gold nanoparticles modified electrode. Microchim Acta 181, 893–901 (2014). https://doi.org/10.1007/s00604-014-1177-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1177-x