Abstract
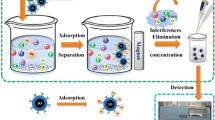
Magnetic Fe3O4@SiO2 core shell nanoparticles containing diphenylcarbazide in the shell were utilized for solid phase extraction of Hg(II) from aqueous solutions. The Hg(II) loaded nanoparticles were then separated by applying an external magnetic field. Adsorbed Hg(II) was desorbed and its concentration determined with a rhodamine-based fluorescent probe. The calibration graph for Hg(II) is linear in the 60 nM to 7.0 μM concentration range, and the detection limit is at 23 nM. The method was applied, with satisfying results, to the determination of Hg(II) in industrial waste water.






Similar content being viewed by others
References
He Q, Mille EW, Wong AP, Chang CJ (2006) A selective fluorescent sensor for detecting lead in living cells. J Am Chem Soc 128:9316–9317
De Silva AP, Gunaratne HQ, Gunnlaugsson NT, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Jønsson JB, Charles E, Kalvig P (2013) How to mitigate mercury pollution in Tanzania. Resour Policy 38:60–67
Ouédraogo O, Amyot M (2013) Mercury, arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Sci Total Environ 444:243–254
Park SK, Lee S, Basu N, Franzblau (2013) Associations of blood and urinary mercury with hypertension in U.S. adults: the Nhanes 2003–2006. Environ Res 123:25–32
Arcagni M, Campbell L, Arribére MA, Marvin-DiPasquale M, Rizzo AS, Guevara R (2013) Mercury arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Sci Total Environ 454:170–180
Savery LC, Evers DC, Wise SS, Falank C, Wise J Jr, Gianios C, Kerr I, Payne R, Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Wise JP Sr (2013) Global mercury and selenium concentrations in skin from free-ranging sperm whales (Physeter macrocephalus). Sci Total Environ 450:59–71
Mercury Update (2001) Impact on fish advisories. EPA Fact Sheet EPA- 823-F-01-011. EPA, Office of Water, Washington, DC
Zhang G, Zhang D, Yin S, Yang X, Shuai Z, Zhu D (2005) 1,3-Dithiole-2-thione derivatives featuring an anthracene unit: new selective chemodosimeters for Hg(II) ion. Chem Commun 2161–2163
Song KC, Kim JS, Park SM, Chung K, Ahn CS, Chang SK (2006) Fluorogenic Hg2+-selective chemodosimeter derived from 8-hydroxyquinoline. Org Lett 8:3413–3416
Sancenón F, Martínez-Máñez, Soto RJ (2001) 1,3,5-Triarylpent-2-en-1,5-diones for the colorimetric sensing of the mercuric cation. Chem Commun 2262–2263
Peng XH, Wang YJ, Tang XL, Liu WS (2011) Functionalized magnetic core-shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II). Dyes Pigments 91:26–32
Zhu M, Yuan M, Liu X, Xu J, Lv J, Huang C (2008) Visible near-infrared chemosensor for mercury ion. Org Lett 10:1481–1484
Coskun A, Yilmaz MD, Akkaya EU (2007) Bis(2-pyridyl)-substituted boratriazaindacene as an NIR-emitting chemosensor for Hg(II). Org Lett 9:607–609
Avirah RR, Jyothish K, Ramaiah D (2007) Dual-mode semisquaraine-based sensor for selective detection of Hg2+ in a micellar medium. Org Lett 9:121–124
Zhang X, Shiraishi Y, Hirai T (2007) Cu(II)-selective green fluorescence of a rhodamine − diacetic acid conjugate. Org Lett 9:5039–5042
Mello JV, Finney NS (2005) Reversing the discovery paradigm: a new approach to the combinatorial discovery of fluorescent chemosensors. J Am Chem Soc 127:10124–10125
Soh JH, Swamy KMK, Kim SK, Kim S, Lee SH, Yoon J (2007) Rhodamine urea derivatives as fluorescent chemosensors for Hg2+. Tetrahedron Lett 48:5966–5969
Wu D, Huang W, Duan C, Lin Z, Meng Q (2007) Highly sensitive fluorescent probe for selective detection of Hg2+ in dmf aqueous media. Inorg Chem 46:1538–1540
Yang YK, Yook KJ, Tae J (2005) Multisignaling optical-electrochemical sensor for Hg2+ based on a rhodamine derivative with a ferrocene unit. J Am Chem Soc 127:16760–16761
Kwon JM, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127:10107–10111
Lee MH, Wu JS, Lee JW, Jung JH, Kim JS (2007) Highly sensitive and selective chemosensor for Hg2+ based on the rhodamine fluorophore. Org Lett 9:2501–2504
Ko SK, Yang YK, Tae J, Shin I (2006) In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J Am Chem Soc 128:14150–14155
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Mao J, Wang LN, Dou W, Tang XL, Yan Y, Liu WS (2007) Tuning the selectivity of two chemosensors to Fe(III) and Cr(III). Org Lett 9:4567–4570
Camel V (2003) Solid phase extraction of trace elements. Spectrochim Acta B 58:1177–1233
Zhai YH, Chang XJ, Cui YM, Lian N, Lai SJ, Zhen H, He Q (2006) Selective determination of trace mercury(II) after preconcentration with 4- (2-pyridylazo)-resorcinol-modified nanometer-sized SiO2 particles from sample solutions. Microchim Acta 154:253–259
Fang G, Tan J, Yan X (2005) An ion-imprinted functionalized silica fel aorbent prepared by a surface imprinting technique combined with a sol–gel process for selective solid-phase extraction of cadmium(II). Anal Chem 77:1734–1739
Mohamed EM, Mahe MO, Mohamed EA (2000) Selective preconcentration and solid phase extraction of mercury(II) from natural water by silica gel-loaded dithizone phases. Anal Chim Acta 415:33–40
Ezzat MS, Mohamed BS, Salwa AA (2004) New solid phase extractors for selective separation and preconcentration of mercury(II) based on silica gel immobilized aliphatic amines 2- thiophenecarboxaldehyde Schiff’s bases. Anal Chim Acta 523:133–140
Ji Y, Liu X, Guan M, Zhao C, Huang H, Zhang Z (2009) A new solid-phase extraction coupled with magnetic carrier technology was developed for extraction of bisphenol A (BPA) and diethylstilbestrol (DES) from water samples. J Sep Sci 32:2139–2145
Shin S, Jang J (2007) Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chem Commun 4230–4232
Huang C, Hu B (2008) Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim Acta B 63:437–444
Takafuji M, Ide S, Ihara H, Xu Z (2004) Preparation of poly(1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal Ions. Chem Mater 16:1977–1983
Wu YW, Zhang J, Liu JF, Chen L, Deng ZL, Han MX, Wei XS, Yu AM, Zhang HL (2012) Fe3O4@ZrO2 nanoparticles magnetic solid phase extraction coupled with flame atomic absorption spectrometry for chromium(III) speciation in environmental and biological samples. Appl Surf Sci 258:6772–6776
Rastkari N, Ahmadkhaniha R (2013) Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of phthalate monoesters in urine samples. J Chromatogr A 1286:22–28
Sadeghi S, Azhdari H, Arabi H, Moghaddam AZ (2012) Surface modified magnetic Fe3O4 nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. J Hazard Mater 215:208–216
Bianchi F, Chiesi V, Casoli F, Luches P, Nasi L, Careri M, Mangia A (2012) Magnetic solid-phase extraction based on diphenyl functionalization of Fe3O4 magnetic nanoparticles for the determination of polycyclic aromatic hydrocarbons in urine samples. J Chromatogr A 1231:8–15
Deng M, Jiang C, Jia L (2013) N-Methylimidazolium modified magnetic particles as adsorbents for solid phase extraction of genomic deoxyribonucleic acid from genetically modified soybeans. Anal Chim Acta 771:31–36
Jiang HM, Yang T, Wang YH, Lian HZ, Hu X (2013) Magnetic solid-phase extraction combined with graphite furnace atomic absorption spectrometry for speciation of Cr(III) and Cr(VI) in environmental waters. Talanta 116:361–367
Zhai YH, Duan SE, He Q, Yang XH, Han Q (2010) Solid phase extraction and preconcentration of trace mercury(II) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim Acta 169:353–360
Khan A, Mahmood F, Khokhar MY, Ahmad S (2006) Functionalized sol–gel material for extraction of mercury (II). React Funct Polym 66:1014–1020
Liang X, Wang X, Zhuang J, Chen Y, Wang D (2006) Synthesis of nearly monodisperse iron oxide and oxyhydroxide nanocrystals. Adv Funct Mater 16:1805–1813
Wu JS, Hwang IC, Kim KS, Kim JS (2007) Rhodamine-based Hg2+-selective chemodosimeter in aqueous solution: fluorescent off − on. Org Lett 9:907–910
Anthoni U, Christophersen C, Nielsen P, Puschl A, Schaumburg K (1995) A new rhodamine B-based lysosomal pH fluorescent indicator. Struct Chem 6:161–165
Gao SF, Tan GQ, Yuan HY, Xiao D, Choi MMF (2006) A simple fluorometric method using chlorophyll a for determination of Hg2+ ion. Microchim Acta 153:159–162
Yang R, Li K, Wang K, Liu F, Li N, Zhao F (2002) Cyclodextrin-porphyrin supramolecular sensitizer for mercury(II) ion. Anal Chim Acta 469:285–293
Chan WH, Yang RH, Wang KM (2001) Development of a mercury ion-selective optical sensor based on fluorescence quenching of 5,10,15,20-tetraphenylporphyrin. Anal Chim Acta 444:261–269
De la Riva BSV, Costa-Fernandez JM, Pereiro R, Sanz-Medel A (2000) Fluorimetric method for the determination of trace levels of mercury in sea water using 6-mercaptopurine. Anal Chim Acta 419:33–40
Wang Z, Zhang D, Zhu D (2005) A sensitive and selective “turn on” fluorescent chemosensor for Hg(II) ion based on a new pyrene–thymine dyad. Anal Chim Acta 549:10–13
Sandor M, Geistmann F, Schuster M (1999) An anthracene-substituted benzoylthiourea for the selective determination of Hg(II) in micellar media. Anal Chim Acta 388:19–26
Acknowledgments
This study was supported by the Natural Science Foundation of Anhui Province (1208085QB34, 2012SQRL148ZD) and Anhui Science and Technology University Key Subject (AKXK20102-2). Thanks Dr. Qun He, and Dr. Yun-Hui Zhai for magnetic core shell material synthesis and characteristics research. Thanks Dr. Zhiwei Zhang, and Dr. En Zhang for NMR measurement and structure analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Electronic Supplementary Material (ESM) available: Synthesis of RP, RP fluorescence response on Hg(II), Job’s plot, pH effect on RP fluorescence, Interference data of potentially interfering ion. This material is available free of charge via the internet at http://link.springer.com/journal/604.
ESM 1
(DOC 1547 kb)
Rights and permissions
About this article
Cite this article
Pan, F., Mao, J., Chen, Q. et al. Solid-phase extraction of mercury(II) with magnetic core-shell nanoparticles, followed by its determination with a rhodamine-based fluorescent probe. Microchim Acta 180, 1471–1477 (2013). https://doi.org/10.1007/s00604-013-1084-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1084-6