Abstract
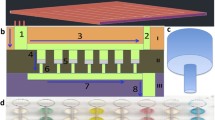
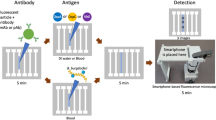
We have developed a sensitive, specific, rapid and low cost picoliter microsphere-based platform for bioanalyte detection and quantification. In this method, a biological sample, biosensing microspheres, and fluorescently labeled detection (secondary) antibodies are co-encapsulated to capture the analyte (here: human anti-tetanus immunoglobulin G) on the surface of the microsphere in microfluidic pL-sized droplets. The absorption of the analyte and detecting antibodies on the microsphere concentrate the fluorescent signal in correlation with analyte concentration. Using our platform and commercially available antibodies, we were able to quantify anti-tetanus antibodies in human serum. In comparison to standard bulk immunosorbent assays, the microfluidic droplet platform presented here reduces the reagent volume by four orders of magnitude, while fast reagent mixing reduces the detection time from hours to minutes. We consider this platform to be a major leap forward in the miniaturization of immunosorbent assays and to provide a rapid and low cost tool for global point-of-care.

We have developed a sensitive, specific, rapid and low cost pico-liter microsphere based platform for detection and quantification of human anti-tetanus immunoglobulin G. In this method, a biological sample, biosensing microspheres, and fluorescently labeled detection antibodies are co-encapsulated to capture the analyte on the surface of the microsphere in microfluidic pL-sized droplets. Using our platform and commercially available antibodies, we quantified the anti-tetanus antibodies content in human serum.





Similar content being viewed by others
References
Pai NP, Vadnais C, Denkinger C, Engel N, Pai M (2012) Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 9(9):e1001306
Mabey DC, Sollis KA, Kelly HA, Benzaken AS, Bitarakwate E, Changalucha J, Chen X-S, Yin Y-P, Garcia PJ, Strasser S, Chintu N, Pang T, Terris-Prestholt F, Sweeney S, Peeling RW (2012) Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS Med 9(6):e1001233
Scott T (2012) Point-of-care tetanus immunoassay: an audit of unscheduled tetanus prophylaxis. Int J Orthop Trauma Nurs 16(2):97–103
Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK (2012) Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med 17(8):1015–1019
Andries A-C, Duong V, Ngan C, Ong S, Huy R, Sroin KK, Te VYB, Try PL, Buchy P (2012) Field evaluation and impact on clinical management of a rapid diagnostic kit that detects dengue NS1, IgM and IgG. PLoS Negl Trop Dis 6(12):e1993
Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NE, Kuchel GA (2006) Elisa and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci 63(8):879–884
Konry T, Bale S, Bhushan A, Shen K, Seker E, Polyak B, Yarmush M (2012) Particles and microfluidics merged: perspectives of highly sensitive diagnostic detection. Microchim Acta 176(3–4):251–269
Wang F, Burns M (2010) Droplet-based microsystem for multi-step bioreactions. Biomed Microdevices 12(3):533–541
Theberge AB, Courtois F, Schaerli Y, Fischlechner M, Abell C, Hollfelder FS, Huck WT (2010) Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew Chem Int Ed 49:5846–5868
Song H, Tice JD, Ismagilov RF (2003) A microfluidic system for controlling reaction networks in time. Angew Chem Int Ed 42(7):768–772
Song H, Chen DL, Ismagilov RF (2006) Reactions in droplets in microfluidic channels. Angew Chem Int Ed 45(44):7336–7356
Sung-Kwon C, Hyejin M, Chang-Jin K (2003) Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J Microelectromech Syst 12(1):70–80
Ahmed R, Jones TB (2006) Dispensing picoliter droplets on substrates using dielectrophoresis. J Electrost 64(7–9):543–549
Lehmann U, Vandevyver C, Parashar VK, Gijs MAM (2006) Droplet-based DNA purification in a magnetic lab-on-a-chip. Angew Chem Int Ed 45(19):3062–3067
Guttenberg Z, Muller H, Habermuller H, Geisbauer A, Pipper J, Felbel J, Kielpinski M, Scriba J, Wixforth A (2005) Planar chip device for PCR and hybridization with surface acoustic wave pump. Lab Chip 5(3):308–317
Wang F, Burns M (2009) Performance of nanoliter-sized droplet-based microfluidic PCR. Biomed Microdevices 11(5):1071–1080
Anna SL, Bontoux N, Stone HA (2003) Formation of dispersions using “flow focusing” in microchannels. Appl Phys Lett 82(3):364–366
Roman OG (2005) Chaotic mixing in thermocapillary-driven microdroplets. Phys Fluids 17(3):033601
Gunther A, Jhunjhunwala M, Thalmann M, Schmidt MA, Jensen KF (2005) Micromixing of miscible liquids in segmented gasliquid flow. Langmuir 21(4):1547–1555
Duda JL, Vrentas JS (1971) Steady flow in the region of closed streamlines in a cylindrical cavity. J Fluid Mech 45(02):247–260. doi:10.1017/S002211207100003X
Handique K, Burns MA (2001) Mathematical modeling of drop mixing in a slit-type microchannel. J Micromech Microeng 11:548–554
Casadevall i Solvas X, deMello A (2012) Droplet microfluidics: recent developments and future applications. Chem Commun 47(7):1936–1942
Guo MT, Rotem A, Heyman JA, Weitz DA (2012) Droplet microfluidics for high-throughput biological assays. Lab Chip 12(12):2146–2155
Konry T, Dominguez-Villar M, Baecher-Allan C, Hafler DA, Yarmush ML (2011) Droplet-based microfluidic platforms for single T cell secretion analysis of IL-10 cytokine. Biosens Bioelectron 26(5):2707–2710
Ogunrin OA (2009) Tetanus—a review of current concepts in management. Benin J Postgrad Med 11(46–61)
Perry AL, Hayes AJ, Cox HA, Alcock F, Parker AR (2009) Comparison of five commercial anti-tetanus toxoid immunoglobulin G enzyme-linked immunosorbent assays. Clin Vaccine Immunol 16(12):1837–1839
Chin CD, Linder V, Sia SK (2012) Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 12(12):2118–2134
Zhu H, Yaglidere O, Su T-W, Tseng D, Ozcan A (2011) Cost-effective and compact wide-field fluorescent imaging on a cell-phone. Lab Chip 11(2):315–322
Acknowledgments
This work was supported in part by the ECOR postdoctoral award to AG and Shriners Grant #85120-BOS. We are also thankful to Dr. Jon F. Edd for the help in design of the array.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golberg, A., Yarmush, M.L. & Konry, T. Picoliter droplet microfluidic immunosorbent platform for point-of-care diagnostics of tetanus. Microchim Acta 180, 855–860 (2013). https://doi.org/10.1007/s00604-013-0998-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-0998-3