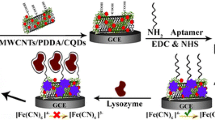
Abstract
We report on a sensitive, simple, label-free impedance-based immunoelectrode for the determination of microcystin-LR (MCLR). The surface of the electrode was modified with a composite made from multiwalled carbon nanotubes and an ionic liquid, and with immobilized polyclonal antibody against MCLR. Cyclic voltammetry and impedance spectroscopy were applied to characterize the modified electrode. It is found that the multi-walled carbon nanotubes act as excellent mediators for the electron transfer between the electrode and dissolved hexacyanoferrate redox pair, while the ionic liquid renders it biocompatible. The method exhibits a wide linear range (0.005 μg•L-1 to 1.0 μg•L-1), a low detection limit (1.7 ng•L-1) and a long-term stability of around 60 days. The ionic liquid 1-amyl-2,3-dimethylimidazolium hexafluorophosphate gave the best impedimetric response. The new immunoelectrode is sensitive, stable, and easily prepared. It has been successfully applied to the determination of MCLR in water samples.

The immunosensor, modified with a nanocomposite of room temperature ionic liquid- multiwalled carbon nanotube, was applied to detect MCLR. The method exhibits a wide linear range (0.005 μg·L−1 to 1.0 μg·L−1), a low detection limit (1.7 ng·L-1) and a long-term stability of around 60 days.





Similar content being viewed by others
References
Dawson RM (1998) The toxicology of microcystins. Toxicon 36:953–962
Žegura B, Sedmak B, Filipič M (2003) Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 41:41–48
Gupta N, Pant SC, Vijayaraghavan R, Rao PVL (2003) Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 188:285–296
McElhiney J, Lawton LA (2005) Detection of the cyanobacterial hepatotoxins microcystins. Toxicol Appl Pharm 203:219–230
Pumera M, Merkoçi A, Alegret S (2006) Carbon nanotube-epoxy composites for electrochemical sensing. Sensors Actuators B: Chem 113:617–622
Xu ZA, Chen X, Qu XH, Dong SJ (2004) Electrocatalytic oxidation of catechol at multi-walled carbon nanotubes modified electrode. Electroanal 16:684–687
WHO (2004) Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva
Mountfort DO, Holland P, Sprosen J (2005) Method for detecting classes of microcystins by combination of protein phosphatase inhibition assay and ELISA: comparison with LC-MS. Toxicon 45:199–206
Herranz S, Bocková M, Marazuela MD, Homola J, Moreno-Bondi MC (2010) An SPR biosensor for the detection of microcystins in drinking water. Anal Bioanal Chem 398:2625–2634
Loyprasert S, Thavarungkul P, Asawatreratanakul P, Wongkittisuksa B, Limsakul C, Kanatharana P (2008) Label-free capacitive immunosensor for microcystin-LR using self-assembled thiourea monolayer incorporated with Ag nanoparticles on gold electrode. Biosens Bioelectron 24:78–86
Chu FS, Huang X, Wei RD (1990) Enzyme-linked immunosorbent assay for microcystins in blue-green algal blooms. J Assoc Off Anal Chem 73:451–457
Khreich N, Lamourette P, Renard PY, Clavé G, Fenaille F, Créminon C, Volland H (2009) A highly sensitive competitive enzyme immunoassay of broad specificity quantifying microcystins and nodularins in water samples. Toxicon 53:551–559
Liu Y, Zhang D, Alocilja EC, Chakrabartty S (2010) Biomolecules detection using a silver-enhanced gold nanoparticle-based biochip. Nanoscale Res Lett 5:533–538
Rubianes MD, Rivas GA (2003) Carbon nanotubes paste electrode. Electrochem Commun 5:689–694
Chen PH, McCreery RL (1996) Control of electron transfer kinetics at glassy carbon electrodes by specific surface modification. Anal Chem 68:3958–3965
Sassolas A, Catanante G, Fournier D, Marty JL (2011) Development of a colorimetric inhibition assay for microcystin-LR detection: comparison of the sensitivity of different protein phosphatases. Talanta 85:2498–2503
Han JH, Zhang JP, Xia YT, Jiang L (2011) Highly sensitive detection of the hepatotoxin microcystin-LR by surface modification and bio-nanotechnology. Colloid Surf A-Physicochem Eng Asp 391:184–189
Barzen C, Brecht A, Gauglitz G (2002) Optical multiple-analyte immunosensor for water pollution control. Biosens Bioelectron 17:289–295
Long F, He M, Zhu AN, Shi HC (2009) Portable optical immunosensor for highly sensitive detection of microcystin-LR in water samples. Biosens Bioelectron 24:2346–2351
Talaty ER, Raja S, Storhaug VJ, Dölle A, Carper WR (2004) Raman and infrared spectra and ab initio calculations of C2-4MIM imidazolium hexafluorophosphate ionic liquids. J Phys Chem B 108:13177–13184
Zhang J, Lei JP, Xu CL, Ding L, Ju HX (2010) Carbon nanohorn sensitized electrochemical immunosensor for rapid detection of microcystin-LR. Anal Chem 82:1117–1122
Cleuziou JP, Wernsdorfer W, Ondarçuhu T, Monthioux M (2011) Electrical detection of individual magnetic nanoparticles encapsulated in carbon nanotubes. ACS Nano 5:2348–2355
Shi F, Deng YQ (2005) Abnormal FT-IR and FTRaman spectra of ionic liquids confined in nano-porous silica gel. Spectrochim Acta A 62:239–244
Shervedani RK, Bagherzadeh M (2009) Electrochemical impedance spectroscopy as a transduction method for electrochemical recognition of zirconium on gold electrode modified with hydroxamated self-assembled monolayer. Sensor Actuat B- Chem 139:657–664
Briza PL, Arben M (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16
Du P, Liu SN, Wu P, Cai CX (2007) Single-walled carbon nanotubes functionalized with poly(Nile blue a) and their application to dehydrogenase-based biosensors. Electrochim Acta 53:1811–1823
Saini RK, Chiang IW, Peng HP, Smalley RE, Billups WE, Hauge RH, Margrave JL (2003) Covalent sidewall functionalization of single wall carbon nanotubes. J Am Chem Soc 125:3617–3621
Ding SF, Xu MQ, Zhao GC, Wei XW (2007) Direct electrochemical response of myoglobin using a room temperature ionic liquid, 1-(2-hydroxyethyl)-3-methyl imidazolium tetrafluoroborate, as supporting electrolyte. Electrochem Commun 9:216–220
Wei D, Kvarnström C, Lindfors T, Ivaska A (2007) Electrochemical functionalization of single walled carbon nanotubes with polyaniline in ionic liquids. Electrochem Commun 9:206–210
Laszlo JA, Compton DL (2002) Comparison of peroxidase activities of hemin, cytochrome c and microperoxidase-11 in molecular solvents and imidazolium-based ionic liquids. J Mol Catal B-Enzym 18:109–120
Dossi N, Toniolo R, Pizzariello A, Carrilho E, Piccin E, Battiston S, Bontempelli G (2012) An electrochemical gas sensor based on paper supported room temperature ionic liquids. Lab Chip 12:153–158
Li ZJ, Wang ZY, Sun XL, Fang YJ, Chen PP (2010) A sensitive and highly stable electrochemical impedance immunosensor based on the formation of silica gel–ionic liquid biocompatible film on the glassy carbon electrode for the determination of aflatoxin B1 in bee pollen. Talanta 80:1632–1637
Tao WY, Pan DW, Liu Q, Yao SZ, Nie Z, Han BX (2006) Optical and bioelectrochemical characterization of water-miscible ionic liquids based composites of multiwalled carbon nanotubes. Electroanal 18:1681–1688
Woody RW (2009) Circular dichroism spectrum of peptides in the poly(pro)II conformation. J Am Chem Soc 131:8234–8245
Xia Y, Zhang J, Jiang L (2011) A novel dendritic surfactant for enhanced microcystin-LR detection by double amplification in a quartz crystal microbalance biosensor. Colloids and Surfaces B: Biointerfaces 86:81–86
Wei Q, Zhao Y, Du B, Wu D, Cai Y, Mao K, Li H, Xu C (2011) Nanoporous PtRu alloy enhanced nonenzymatic immunosensor for ultrasensitive detection of microcystin-LR. Adv Funct Mater 21:4193–4198
Queirós RB, Silva SO, Noronha JP, Frazão O, Jorge P, Aguilar G, Marques PVS, Sales MGF (2011) Microcystin-LR detection in water by the fabry–pérot interferometer using an optical fibre coated with a sol–gel imprinted sensing membrane. Biosens Bioelectron 26:3932–3937
Tian J, Zhao H, Zhao H, Quan X (2012) Photoelectrochemical immunoassay for microcystin-LR based on a fluorine-doped tin oxide glass electrode modified with a CdS-graphene composite. Microchim Acta 179:163–170
Acknowledgments
This work has been supported by the “973” National Basic Research Program of China (No. 2012CB720804), National research program (No. 2011BAK10B03, No. 201203069–1/No. 201003008-08-03), and Fundamental Research Funds for the Central Universities, (JUSRP21101.)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 181 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Guan, L., Shi, H. et al. Determination of microcystin-LR with a glassy carbon impedimetric immunoelectrode modified with an ionic liquid and multiwalled carbon nanotubes. Microchim Acta 180, 75–83 (2013). https://doi.org/10.1007/s00604-012-0912-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0912-4