Abstract
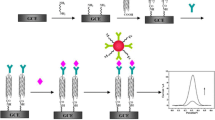
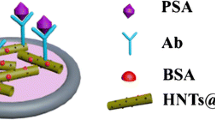
We reported on a novel electrochemical immunosensor for the determination of prostate specific antigen (PSA). It is based on gold nanoparticles supported on cross-linked starch functionalized multi-walled carbon nanotubes in a nanocomposite film. The method is simple and can be carried out at room temperature without the use of expensive chemicals or corrosive acids. Thus, it preserves the integrity and the electronic structure of the nanotubes. The stepwise assembly of the electrode was characterized by cyclic voltammetry (CV) and impedance spectroscopy. CV studies demonstrated that the formation of antibody-antigen complex decreases the peak current of the hexacyanoferrate redox pair linearly with increasing PSA concentration in two ranges (0.01–0.5 and 0.5–3.0 ng·mL−1). The detection limit for PSA is 7 pg·mL−1 (at S/N = 3). This immunoassay exhibits good sensitivity and reproducibility and may become a promising technique for the diagnosis and monitoring of prostate cancer.

A novel electrochemical immunosensor for the detection of prostate specific antigen (PSA) based on Au nanoparticles supported on cross-linked starch-functionalized multi-walled carbon nanotubes(AuNPs/MWCNTs–CAS) nanocomposite film was developed.





Similar content being viewed by others
References
Qu B, Chu X, Shen GL, Yu RQ (2008) A novel electrochemical immunosensor based on colabeled silica nanoparticles for determination of total prostate specific antigen in human serum. Talanta 76:785–790
Panini NV, Messina GA, Salinas E, Fernández H, Raba J (2008) An amperometric biosensor based on hemoglobin immobilized in poly(epsilon-caprolactone) film and its application. Biosens Bioelectron 23:1145–1151
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E (1987) Prostate-specificantigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317:909–916
Institute of Isotopes Ltd. Technical Bulletin for PSA 125I IRMA kit. http://www.izotop.hu/?page_id=31#
Diagnostic Systems Laboratories, Inc. Technical Bulletin for Avtive PSA ELISA vs Hybritech Tamdom PSA IRMA. http://www.dslabs.com
Goldsmith SJ (1975) Radioimmunoassay: review of basic principles. Semin Nucl Med 5:125–152
Teppo AM, Maury CPJ (1987) Radioimmunoassay of tumor necrosis factor in serum. Clin Chem 33:2024–2027
Zheng Y, Chen H, Liu XP, Jiang JH, Luo Y, Shen GL, Yu RQ (2008) An ultrasensitive chemiluminscence immunosensor for PSA based on theenzyme encapsulated liposome. Talanta 77:809–814
Fu ZF, Yan F, Liu H, Yang ZJ, Ju HX (2008) Channel-resolved multianalyte immunosensing system for flow-through chemiluminescent detection of α-fetoprotein and carcinoembryonic antigen. Biosens Bioelectron 23:1063–1069
Brian CM, Sinang C, Leslie RF, Melisenda JM (2010) Polymer–protein-enhanced fluoroimmunoassay for prostate-specific antigen. Anal Bioanal Chem 396(2):681–686
Voller A, Bartlett A, Bidwell DE (1978) Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol 31:507–520
Matsuya T, Tashiro S, Hoshino N, Shibata N, Nagasaki Y, Kataoka K (2003) A core-shell type fluorescent nanosphere possessing reactive poly(ethylene glycol) tethered chains on the surface of zeptomole detection of protein in time-resolved fluorometric immunoassay. Anal Chem 75:6124–6132
Cesaro-Tadic S, Dernick G, Juncker D, Buurman G, Kropshofer H, Michel B, Fattinger C, Delamarche E (2004) High-sensitivity miniaturized immunoassays for tumor necrosis factor α using microfluidic systems. Lab Chip 4:563–569
Schmalzing D, Nashabeh W (1997) Capillary electrophoresis based immunoassays: a critical review. Electrophoresis 18:2184–2193
Niederkofler EE, Tubbs KA, Gruber K, Nedelkov D, Kiernan UA, Williams P, Nelson RW (2001) Determination of beta-2 microglobulin levels in plasma using a high-throughput mass spectrometric immunoassay system. Anal Chem 73:3294–3299
Hu SH, Zhang SC, Hu ZC, Xing Z, Zhang XR (2007) Detection of multiple proteins on one spot by laser ablation inductively coupled plasma mass spectrometry and application to immuno- microarray with element-tagged antibodies. Anal Chem 79:923–929
Saito K, Kobayashi D, Sasaki M, Araake H, Kida T, Yagihashi A, Yajima T, Kameshima H, Watanabe N (1999) Detection of human serum tumor necrosis factor-alpha in healthy donors, using a highly sensitive immuno-PCR assay. Clin Chem 45:665–669
Wang GF, Huang H, Zhang G, Zhang XJ, Fang B, Wang L (2011) Dual amplification strategy for the fabrication of highly sensitive interleukin-6 amperometric immunosensor based on poly-dopamine. Langmuir 27:1224–1231
Liu ZY, Yuan R, Chai YQ, Zhuo Y, Hong CL (2009) Highly sensitive reagentless amperometric immunosensor based on layer-by-layer assembly of redox-active organic–inorganic composite film for determining prostate specific antigen. Acat Chimica Sinica 67(7):637–644
Ning Z, He YQ, Mao X, Sun YH, Zhang XB, Li CZ, Lin YH, Liu GD (2010) Electrochemical assay of active prostate-specific antigen (PSA) using ferrocene-functionalized peptide probes. Electrochem Commun 12:471–474
Kim DJ, Lee NE, Park JS, Park IJ, Kim JG, Cho HJ (2010) Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens Bioelectron 25:2477–2482
Yin ZZ, Cui RJ, Liu Y, Jiang LP, Zhu JJ (2010) Ultrasensitive electrochemical immunoassay based on cadmium ion-functionalized PSA@PAA nanospheres. Biosens Bioelectron 25:1319–1324
Mi Q, Wang ZW, Chai CY, Zhang J, Zhao B, Chen CY (2011) Multilayer structured immunosensor based on a glassy carbon electrode modified with multi-wall carbon nanotubes, polythionine, and gold nanoparticles. Microchim Acta 173:459–467
Merkoci A (2006) Carbon nanotubes in analytical sciences. Microchim Acta 152:157–174
Guo G, Qin F, Yang D (2008) Synthesis of platinum nanoparticles supported on Poly(acrylic acid) grafted MWNTs and their hydrogenation of citral. J Chem Mater 20:2291–2297
Feng XM, Hu JQ, Chen XH, Xie JS, Liu YY (2009) Synthesis and electron transfer property of sulfhydryl-containing multi-walled carbon nanotube/gold nanoparticleheterojunctions. Appl Phys 42:042001–042006
Sardesai N, Pan SM, Rusling J (2009) Electrochemiluminescent immunosensor for detection of protein cancer biomarkers using carbon nanotube forests and [Ru-(bpy)3]2+-doped silica nanoparticles. Chem Commun 33:4968–4970
Okuno K, Maehashi K, Kerman K, Takamura Y, Matsumoto K, Tamiya E (2007) Label-free immunosensor for prostate-specific antigen based on single-walled carbon nanotube array-modified microelectrodes. Biosens Bioelectron 22:2377–2381
Wildgoose GG, Banks CE, Leventis HC, Compton RG (2006) Chemically modified carbon nanotubes for use in electroanalysis. Microchim Acta 152:187–214
Huang KJ, Niu DJ, Xie WZ, Wang W (2010) A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes–chitosans nanocomposite film modified glassy carbon electrode. Anal Chim Acta 659:102–108
Cheng R, Ou S, Li M (2009) Ethylenediamine modified starch as biosorbent for acid dyes. J Hazard Mater 172:1665–1670
Zhao YC, Yang XL, Zhan L, Ou SJ, Tian JN (2011) High electrocatalytic activity of PtRu nanoparticles supported on starch-functionalized multi-walled carbon nanotubes for ethanol oxidation. J Mater Chem 21:4257–4263
Hinrichs W, Buttner G, Steifa M, Betzel C, Zabel V, Pfannemuller B, Saenger W, Hinrichs W, Buttner G, Steifa M (1987) An amylose antiparallel double helix at atomic resolution. Science 238:205–208
Star A, Steuerman DW, Heath JR, Stoddart JF (2002) Starched carbon nanotubes. Angew Chem Int Ed 41:2508–2512
Wang S, Wang X, Jiang SP (2008) PtRu nanoparticles supported on 1-Aminopyrene-Functionalized multiwalled carbon nanotubes and their electrocatalytic activity for methanol oxidation. Langmuir 24:10505–10512
Zhao YC, Yang XL, Tian JN, Wang FY, Zhan L (2010) A facile and novel approach toward synthetic polypyrrole oligomers functionalization of multi-walled carbon nanotubes as PtRu catalyst support for methanol electro-oxidation. J Power Sources 195:4634–4640
Li T, Yang MH, Li H (2011) Label-free electrochemical detection of cancer marker based on graphene-cobalt hexacyanoferrate nanocomposite. J Electroanal Chem 655:50–55
Wei Q, Xiang Z, He J, Wang GL, Li H, Qian ZY, Yang MH (2010) Dumbbell-like Au-Fe3O4 nanoparticles as label for the preparation of electrochemical immunosensors. Biosens Bioelectron 26:627–631
Acknowledgments
This work has been supported by National Natural Science Foundation of China (No. 21165004, 21163002), the Guangxi Natural Science Foundation of China (2010GXNSFF013001, 0728043), Innovation Plan in Graduate Education of Guangxi Province (2010106020703 M70) and the project of Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources(Guangxi Normal University), Ministry of Education of China(CMEMR2011-14).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 396 kb)
Rights and permissions
About this article
Cite this article
Tian, J., Huang, J., Zhao, Y. et al. Electrochemical immunosensor for prostate-specific antigen using a glassy carbon electrode modified with a nanocomposite containing gold nanoparticles supported with starch-functionalized multi-walled carbon nanotubes. Microchim Acta 178, 81–88 (2012). https://doi.org/10.1007/s00604-012-0816-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0816-3