Abstract
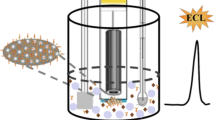
We report on a new scheme for the determination of the activity of caspase-3 using a specific peptide labeled with N-(4-aminobutyl)-N-ethylisoluminol (ABEI) as a chemiluminescent (CL) probe and on the development of magnetic separation technology. Firstly, the ABEI-labeled and biotinylated peptide was prepared and conjugated to streptavidin-coated magnetic beads (MBs) to form f-MBs (functionalized magnetic beads). The f-MBs contain a site (DEVD, Asp-Glu-Val-Asp) that is cleaved by caspase-3. Upon cleavage, the terminal residue attached to ABEI can dissociate from the f-MBs and can be used for CL detection. CL intensity is linearly related to the concentration of caspase-3 in the range 1.0 to 600 ng mL−1, with a detection limit of 0.3 ng mL−1. The relative standard deviation of the assay is 3.6 % at a level of 50 ng mL−1 of caspase-3 (for n = 11). The CL assay has been applied to the determination of caspase-3 in Jurkat cell extract with recoveries between 96.6 % and 106.1 % (n = 5).

A chemiluminescence assay for the detection of caspase-3 activity using N-(4-aminobutyl)-N-ethylisoluminol labeled specific peptide as CL probe coupling the magnetic separation technology was developed. The developed method has been applied to determination of caspase-3 in Jurkat cells extract with a satisfactory.




Similar content being viewed by others
References
Bredesen DE (2008) Programmed cell death mechanisms in neurological disease. Curr Mol Med 8:173–186
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Salvesen GS, Riedl SJ (2008) Caspase mechanisms. Adv Exp Med Biol 615:13–23
Chen N, Huang Y, Yang LL, Liu RH, Yang JJ (2009) Designing caspase–3 sensors for imaging of apoptosis in living cells. Chem Eur J 15:9311–9314
Zhang JJ, Zheng TT, Cheng FF, Zhu JJ (2011) Electrochemical sensing for caspase 3 activity and inhibition using quantum dot functionalized carbon nanotube labels. Chem Commun 47:1178–1180
Ji C, Amarnath V, Pietenpol JA, Marnett LJ (2001) 4-hydroxynonenal induces apoptosis via caspase-3 activation and cytochrome c release. Chem Res Toxicol 14:1090–1096
Gurtu V, Kain SR, Zhang G (1997) Fluorimetric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem 251:98–102
Belloc F, Belaud-Rotureau MA, Lavignolle V, Bascans E, Braz-Pereira E, Durrieu F, Lacombe F (2000) Flow cytometry detection of caspase 3 activation in preapoptotic leukemic cells. Cytometry 40:151–160
Kohler C, Orrenius S, Zhivotovsky B (2002) Measuring immunity: basic biology and clinical assessment. J Immunol Methods 265:97–110
Kim K, Lee M, Park H, Kim JH, Kim S, Chung H, Choi K, Kim IS, Seong BL, Kwon IC (2006) Cell–permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J Am Chem Soc 128:3490–3491
Xiao H, Liu L, Meng FB, Huang JY, Li GX (2008) Electrochemical approach to detect apoptosis. Anal Chem 80:5272–5275
Boeneman K, Mei BC, Dennis AM, Bao G, Deschamps JR, Mattoussi H, Medintz IL (2009) Sensing caspase 3 activity with quantum dot–fluorescent protein assemblies. J Am Chem Soc 131:3828–3829
Prasuhn DE, Feltz A, Blanco-Canosa JB, Susumu K, Stewart MH, Mei BC, Yakovlev AV, Loukov C, Mallet JM, Oheim M, Dawson PE, Medintz IL (2010) Quantum dot peptide biosensors for monitoring caspase 3 proteolysis and calcium ions. ACS Nano 4(9):5487–5497
Zauner T, Berger-Hoffmann R, Muller K, Hoffmann R, Zuchner T (2011) Highly adaptable and sensitive protease assay based on fluorescence resonance energy transfer. Anal Chem 83:7356–7363
Kihara T, Nakamura C, Suzuki M, Han SW, Fukazawa K, Ishihara K, Miyake J (2009) Development of a method to evaluate caspase–3 activity in a single cell using a nanoneedle and a fluorescent probe. Biosens Bioelectron 25:22–27
Fletcher P, Andrew KN, Calokerinos AC, Forbes S, Worsfold PJ (2011) Analytical applications of flow injection with chemiluminescence detection–a review. Luminescence 16:1–23
Hun X, Wang ZP (2011) L–Argininamide biosensor based on S1 nuclease hydrolysis signal amplification. Microchim Acta 176:209–216
Cai D, Ren L, Zhao HZ, Xu CJ, Zhang L, Yu Y et al (2010) A molecular imprint nanosensor for ultrasensitive detection of proteins. Anal Chem 82:241–249
Wu J, Fu XC, Xie CG, Yang M, Fang W, Gao S (2011) TiO2 nanoparticles-enhanced luminol chemiluminescence and its analytical applications in organophosphate pesticide imprinting. Sensor Actuators B 160:511–516
Xie CG, Zhou HK, Gao S, Li HF (2010) Molecular imprinting method for on-line enrichment and chemiluminescent detection of the organophosphate pesticide triazophos. Microchim Acta 171:355–362
Kulmala S, Suomi J (2003) Current status of modern analytical luminescence methods. Anal Chim Acta 500:21–69
Gunn DL, Roszman TL (1972) Preparation of sensitive and stable erythrocytes by the carbodiimide method for the detection of primary and secondary IgM and IgG antibody. J Immunol Methods 1:381–389
Hun X, Chen HC, Wang W (2010) Design of ultrasensitive chemiluminescence detection of lysozyme in cancer cells based on nicking endonuclease signal amplification technology. Biosensor Bioelectron 26:248–254
Albarran B, To R, Stayton PS (2005) A TAT–streptavidin fusion protein directs uptake of biotinylated cargo into mammalian cells. Protein Eng Des Sel 18(3):147–152
Wang HB, Zhang Q, Chu X, Chen TT, Ge J, Yu RQ (2011) G raphene oxide–peptide conjugate as an intracellular protease sensor for caspase–3 activation imaging in live cells. Angew Chem Int Ed 50:7065–7069
Burdo TG, Seltz WR (1975) Mechanism of cobalt catalysis of luminol chemiluminescence. Anal Chem 47:1639–1643
Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW (1997) Substrate specificities of caspase family proteases. J Biol Chem 272:9677–9682
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 111 kb)
Rights and permissions
About this article
Cite this article
Li, Y. Chemiluminescent determination of the activity of caspase-3 using a specific peptide substrate and magnetic beads. Microchim Acta 177, 443–447 (2012). https://doi.org/10.1007/s00604-012-0798-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0798-1