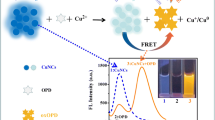
Abstract
We have synthesized the near-infrared water-soluble conjugated polymer poly[2,5-di(propyloxysulfonate)-1,4-phenylene-ethynylene-9,10-anthrylene (referred to as PPEASO3). Its fluorescence (at wavelengths between 650 and 800 nm following photoexcitation at 550 nm) is efficiently quenched by Cu(II) ions, while other physiologically relevant metal ions do not cause significant quenching at the same concentrations. Under optimum conditions, fluorescence intensity is inversely proportional to the concentration of Cu (II). The calibration curve displays two linear regions over the range of 0–3.2 × 10−7 mol L−1 and 3.2 × 10−7 mol L−1 to 1.0 × 10−4 mol L−1 of Cu(II), respectively. The long-wavelength excitation and emission can substantially reduce interferences by the autofluorescence and light scattering of biological matter under UV excitation. The method was successfully applied to the determination of Cu(II) in synthetic and tea samples.

Highly sensitive fluorescent sensor with low background interference was successfully applied to the determination of Cu (II) in synthetic and real samples, based on amplified fluorescence quenching of a water-soluble NIR emitting conjugated polymer.







Similar content being viewed by others
References
Zheng YJ, Huo Q, Kele P, Andreopoulos FM, Pham SM, Leblanc RM (2001) A new fluorescent chemosensor for copper ions based on tripeptide glycyl-histidyl-lysine (GHK). Org Lett 3:3277–3280
Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J (1993) Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper–transporting ATPase. Nat Genet 3:7–13
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 128:10–11
Tong A, Akama Y, Tanaka S (1990) Pre-concentration of copper, cobalt and nickel with 3-methyl-1-phenyl-4-stearoyl-5-pyrazolone loaded on silica gel. Analyst 115:947–949
Budziak D, Silva EL, Campos SD, Carasek E (2003) Application of Nb2O5–SiO2 in pre-concentration and determination of copper and cadmium by flow system with flame atomic absorption spectrometry. Microchim Acta 141:169–174
Tian YH, Pepelnik R, Fanger HV (1990) Multielement analysis of archaic Chinese bronze and antique coins by fast neutron activation analysis. J Radioanal Nucl Chem 139:43–53
Chandra Rao GP, Seshaiah K, Rao YK, Wang MC (2006) Solid phase extraction of Cd, Cu, and Ni from leafy vegetables and plant leaves using amberlite XAD-2 functionalized with 2-hydroxy-acetophenone-thiosemicarbazone (HAPTSC) and determination by inductively coupled plasma atomic emission spectroscopy. J Agric Food Chem 54:2868–2872
Jackson KW, Mahmood TM (1994) Atomic absorption, atomic emission, and flame emission spectrometry. Anal Chem 66:252R–279R
Pournaghi-Azar MH, Dastangoo H (2000) Differential pulse anodic stripping voltammetry of copper in dichloromethane: application to the analysis of human hair. Anal Chim Acta 405:135–144
Poursaberi T, Hajiagha-Babaei L, Yousefi M, Rouhani S, Shamsipur M, Kargar-Razi M, Moghimi A, Aghabozorg H, Ganjali MR (2001) The synthesis of a new thiophene-derivative Schiff’s base and its use in preparation of copper-ion selective electrodes. Electroanal 13:1513–1577
Lai SJ, Chang XJ, Fu C (2009) Cadmium sulfide quantum dots modified by chitosan as fluorescence probe for copper (II) ion determination. Microchim Acta 165:39–44
Luo Y, Li Y, Lv BQ, Zhou ZD, Xiao D, Choi MMF (2009) A new luminol derivative as a fluorescent probe for trace analysis of copper(II). Microchim Acta 164:411–417
Seitz WR (1984) Chemical sensors based on fiber optics. Anal Chem 56:16A–34A
Mei L, Xiang Y, Li N, Tong AJ (2007) A new fluorescent probe of rhodamine B derivative for the detection of copper ion. Talanta 72:1717–1722
Aksuner N, Henden E, Yilmaz I, Cukurovali A (2009) A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a schiff base. Dyes Pigm 83:211–217
Liu Y, Ogawa K, Schanze KS (2008) Conjugated polyelectrolyte based real-time fluorescence assay for phospholipase C. Anal Chem 80:150–158
Liu Y, Zong LL, Zheng LF, Wu LL, Cheng YX (2007) Fluorescent chemosensor for metal ions based on optically active polybinaphthyls and 1,3,4-oxadiazole. Polymer 48:6799–6807
Zeng DL, Chen JG, Ren SJ, Fang Q (2008) A new sensor for copper (II) ion based on carboxyl acid groups substituted polyfluoreneethynylene. React Funct Polym 68:1715–1721
Banjoko V, Xu YQ, Mintz E, Pang Y (2009) Synthesis of terpyridine- functionalized poly(phenylenevinylene)s: the role of meta-phenylene linkage on the Cu2+ and Zn2+ chemosensors. Polymer 50:2001–2009
Thomas SW, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386
He F, Tang Y, Wang S, Li Y, Zhu D (2005) Fluorescent amplifying recognition for DNA G-quadruplex folding with a cationic conjugated polymer: a platform for homogeneous potassium detection. J Am Chem Soc 127:12343–12346
Zhang T, Fan HL, Liu GL, Jiang J, Zhou JG, Jin QH (2008) Different effects of Fe2+ and Fe3+ on conjugated polymer PPESO3: A novel platform for sensitive assays of hydrogen peroxide and glucose. Chem Commun 42:5414–5416
Nelson TL, O’Sullivan C, Greene NT, Maynor MS, Lavigne JJ (2006) Cross-reactive conjugated polymers: analyte-specific aggregative response for structurally similar diamines. J Am Chem Soc 128:5640–5641
Tan C, Atas E, Müller JG, Pinto MR, Kleiman VD, Schanze KS (2004) Amplified quenching of a conjugated polyelectrolyte by cyanine dyes. J Am Chem Soc 126:13685–13694
Xing CF, Shi ZQ, Yu MH, Wang S (2008) Cationic conjugated polyelectrolyte- based fluorometric detection of copper(II) ions in aqueous solution. Polymer 49:2698–2703
Trojanowicz M (2003) Application of conducting polymers in chemical analysis. Microchim Acta 143:75–91
Compagnone D, Ricci A, Del Carlo M, Chiarini M, Pepe A, Sterzo CL (2010) New poly(aryleneethynylene)s as optical active platforms in biosensing. Selective fluorescent detection of Hg(II) obtained by the use of aminoacidic groups anchored on conjugated backbones. Microchim Acta 170:313–319
Zhang T, Fan HL, Zhou JG, Jin QH (2006) Fluorescent conjugated polymer PPESO3: a novel synthetic route and the application for sensing protease activities. Macromolecules 39:7839–7843
Kobayashi E, Jiang J, Ohta H, Furukawa J (1990) Novel conjugated polymer containing anthracene backbone: addition polymer of 9, 10-diethynylanthracene with 9, 10-anthracenedithiol and its properties. J Polym Sci Part A Polym Chem 28:2641–2650
Kato T, Tokuya T, Nozaki T, Takahashi A (1984) Molecular characterization of sodium poly(acrylate) by an aqueous g.p.c./LS method. Polymer 25:218–224
McCormick CL, Callais PA, Hutchinson BH Jr (1985) Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules 18:2394–2401
Itou S, Nishioka N, Norisuye T, Teramoto A (1981) Rodlike nature of.alpha.- helical polypeptides in solution. Macromolecules 14:904–909
Tan C, Pinto MR, Schanze KS (2002) Photophysics, aggregation and amplified quenching of a water-soluble poly(phenylene ethynylene). Chem Commun 36:446–447
Lakowicz JR (1999) Principles of fluorescence spectroscopy. Kluwer Academic/Plenum Publishers, New York
Fan LJ, Zhang Y, Murphy CB, Jones WE Jr (2009) Fluorescent conjugated polymer molecular wire chemosensors for transition metal ion recognition and signaling. Coord Chem Rev 253:410–422
Chen YG, Zhao D, He ZK, Ai XP (2007) Fluorescence quenching of water-soluble conjugated polymer by metal cations and its application in sensor. Spectrochimica Acta Part A 66:448–452
Oehme I, Wolfbeis OS (1997) Optical sensors for determination of heavy metal ions. Mikrochim Acta 126:177–192
Oehme I, Prokes B, Murkovic I, Werner T, Klimant I, Wolfbeis OS (1994) LED-compatible copper(II)-selective optrode membrane based on lipophilized Zincon. Fresenius J Anal Chem 350:563–567
Oehme I, Prattes S, Wolfbeis OS, Mohr GJ (1998) The effect of polymeric supports and methods of immobilization on the performance of an optical copper(II)-sensitive membrane based on the colorimetric reagent Zincon. Talanta 47:595–604
Cano-Raya C, Fernández-Ramos MD, Capitán-Vallvey LF, Wolfbeis OS, Schaferling M (2005) Fluorescence quenching of the europium tetracycline hydrogen peroxide complex by copper(II) and other metal ions. Appl Spectrosc 59:1209–1216
Leth S, Maltoni S, Simkus R, Mattiasson B, Corbisier P, Klimant I, Wolfbeis OS, Csoregi E (2002) Engineered bacteria based biosensors for monitoring bioavailable heavy metals. Electroanalysis 14:35–42
Mayr T, Klimant I, Wolfbeis OS, Werner T (2002) Dual lifetime referenced optical sensor membrane for the determination of copper(II) ions. Anal Chim Acta 462:1–10
Chen B, Zhong P (2005) A new determining method of copper(II) ions at ng ml−1 levels based on quenching of the water-soluble nanocrystals fluorescence. Anal Bioanal Chem 381:986–992
Zhang Y, Zhang H, Guo X, Wang H (2008) L-Cysteine-coated CdSe/CdS core-shell quantum dots as selective fluorescence probe for copper(II) determination. Microchem J 89:142–147
Lai Y, Yu Y, Zhong P, Wu J (2006) Development of novel quantum dots as fluorescent sensors for application in highly sensitive spectrofluorimetric determination of Cu2+. Anal Lett 39:1201–1209
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20875003) and the Natural Science Foundation of Anhui Province (No. 070416239).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 347 kb)
Rights and permissions
About this article
Cite this article
Sun, L., Hao, D., Shen, W. et al. Highly sensitive fluorescent sensor for copper (II) based on amplified fluorescence quenching of a water-soluble NIR emitting conjugated polymer. Microchim Acta 177, 357–364 (2012). https://doi.org/10.1007/s00604-012-0781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0781-x