Abstract
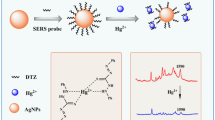
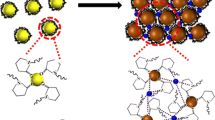
Silver nanoparticles (Ag NPs) modified with sodium 2-mercaptoethanesulfonate (mesna) exhibit strong surface-enhanced Raman scattering (SERS). Their specific and strong interaction with heavy metal ions led to a label-free assay for Hg(II). The covalent bond formed between mercury and sulfur is stronger than the one between silver and sulfur and thus prevents the adsorption of mesna on the surface of Ag NPs. This results in a decrease of the intensity of SERS in the presence of Hg(II) ions. The Raman peak at 795 cm−1 can be used for quantification. The effect of the concentration of mesna, the concentration of sodium chloride, incubation time and pH value on SERS were optimized. Under the optimal conditions, the intensity of SERS decreases with increasing concentration of Hg(II). The decrease is linear in the 0.01 and 2 μmol L−1 concentration range, with a correlation coefficient (R2) of 0.996 and detection limit (S/N = 3) is 0.0024 μmol L−1. The method was successfully applied to the determination of the Hg(II) in spiked water samples.

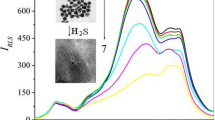
SERS spectra of mesna-Ag NPs system in the presence of Hg2+. Concentrations of Hg2+: (1) 0.1×10-7, (2) 1×10-7, (3) 3.5×10-7, (4) 5×10-7, (5) 12×10-7, (6) 20×10-7mol L-1






Similar content being viewed by others
References
Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP (2005) A blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999–2000. Environ Res 97:195–200
Virtanen JK, Rissanen TH, Voutilainen S, Tuomainen TP (2007) Mercury as a risk factor for cardiovascular diseases. J Nutr Biochem 18:75–85
Yang H, Xu R, Xue X, Li F, Li G (2008) Hybrid surfactant-templated mesoporous silica formed in ethanol and its application for heavy metal removal. J Hazard Mater 152:690–698
Wu ShJ, Li FT, Wu YN, Xu R, Li GT (2010) Preparation of novel poly(vinyl alcohol)/SiO2 composite nanofiber membranes with mesostructure and their application for removal of Cu2+ from waste water. Chem Commun 46:1694–1696
Li YF, Chen ChY, Li B, Sun J, Wang JX, Gao YX, Zhao YL, Chai ZhF (2006) Elimination effciency of different reagents for the memory effect of mercury using ICP-MS. J Anal At Spectrom 21:94–96
Moores A, Goettmann F (2006) The plasmon band in noble metal nanoparticles: an introduction to theory and applications. New J Chem 30:1121–1132
Kim YJ, Johnson RC, Hupp JT (2001) Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett 1:165–167
Chansuvarn W, Imyim A (2011) Visual and colorimetric detection of mercury(II) ion using gold nanoparticles stabilized with a dithia-diaza ligand. Microchim Acta. doi:10.1007/s00604-011-0691-3
Leng B, Zou L, Jiang JB, Tian H (2009) Colorimetric detection of mercuric ion (Hg2+) in aqueous media using chemodosimeter-functionalized gold nanoparticles. Sens A actuators B 140:162–169
Wang YL, Li D, Ren W, Liu ZJ, Dong ShJ, Wang EK (2008) Ultrasensitive colorimetric detection of protein by aptamer–Au nanoparticles conjugates based on a dot-blot assay. Chem Commun 2520–2522
Thomas JM, Ting R, Perrin DM (2004) High affinity DNA zyme-based ligands for transition metal cations–a prototype sensor for Hg2+. Org Biomol Chem 2:307–312
Ono A, Togashi H (2004) Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew Chem Int Ed 43:4300–4302
Li T, Zhou YY, Sun JY, Tang DB, Guo SX, Ding XP (2011) Ultrasensitive detection of mercury(II) ion using CdTe quantum dots in sol-gel-derived silica spheres coated with calix[6]arene as fluorescent probes. Microchim Acta 175:113–119
Weng YM, Weng RH, Tzeng CY, Chen WL (2003) Structural analysis of triacylglycerols and edible oils by near-infrared Fourier transform Raman spectroscopy. Appl Spectrosc 57:413–418
Muik B, Lendl B, Diaz AM, Ayora-Cañada MJ (2003) Direct reagent-free determination of free fatty acid content in olive oil and olives by Fourier transform Raman spectrometry. Anal Chim Acta 487:211–220
Ellis DI, Broadhurst D, Clarke SJ, Goodacre R (2005) Rapid identification of closely related muscle foods by vibrational spectroscopy and machine learning. Analyst 130:1648–1654
Mulvihill M, Tao A, Benjauthrit K, Arnold J, Yang PD (2008) Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water. Angew Chem Int Ed 47:6456–6460
Jana BNR, Pal T (2007) Anisotropic metal nanoparticles for use as surface-enhanced Raman substrates. Adv Mater 19:1761–1765
Le Ru EC, Etchegoin PG, Meyer M (2006) Enhancement factor distribution around a single surface-enhanced Raman scattering hot spot and its relation to single molecule detection. J Chem Phys 125:204701–204713
Camden JP, Dieringer JA, Wang YM, Masiello DJ, Marks LD, Schatz GC, Van Duyne RP (2008) Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J Am Chem Soc 130:12616–12617
Lee SJ, Morrill AR, Moskovits M (2006) Hot spots in silver nanowire bundles for surface-enhanced Raman spectroscopy. J Am Chem Soc 128:2200–2201
Imura K, Okamoto H, Hossain MK, Kitajima M (2006) Visualization of localized intense optical fields in single gold−nanoparticle assemblies and ultrasensitive Raman active sites. Nano Lett 6:2173–2176
Luo ZhH, Chen K, Lu DL, Han HY, Zou MQ (2011) Synthesis of p-aminothiophenol-embedded gold/silver core-shell nanostructures as novel SERS tags for biosensing applications. Microchim Acta 173:149–156
Yu XJ, Jiang ZhH, Wang QJ, Guo YSh (2010) Silver nanoparticle-based chemiluminescence enhancement for the determination of norfloxacin. Microchim Acta 171:17–22
Kim K, Park HK, Kim NH (2006) Silver-particle-based surface-enhanced Raman scattering spectroscopy for biomolecular sensing and recognition. Langmuir 22:3421–3427
Bogdanchikova NE, Kurbatov AV, Tret’yakov VV, Rodionov PP (1992) Activity of colloidal silver preparations towards smallpox virus. Pharm Chem J 26:778–779
Pietrobon B, McEachran M, Kitaev V (2009) Synthesis of size-controlled faceted pentagonal silver nanorods with tunable plasmonic properties and self-assembly of these nanorods. ACS Nano 3:21–26
Dieringer JA, McFarland AD, Shah NC, Stuart DA, Whitney AV, Yonzon CR, Young MA, Zhang X, Van Duyne RP (2006) Surface enhanced Raman spectroscopy: new materials, concepts, characterization tools, and applications. Faraday Discuss 132:9–26
Moskovits M (1985) Surface-enhanced spectroscopy. Rev Mod Phys 57:783–826
Otto A, Mrozek I, Grabhorn H, Akemann W (1992) Surface-enhanced Raman scattering. J Phys Condens Matter 4:1143–1212
Lee PC, Meise D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391–3395
Kudelski A (2002) Raman and electrochemical characterization of 2-mercaptoethanesulfonate monolayers on silver: a comparison with monolayers of 3-mercaptopropionic acid. Langmuir 18:4741–4747
Kudelski A, Pecul M, Bukowska J (2002) Interaction of 2-mercaptoethanesulfonate monolayers on silver with sodium cations. J Raman Spectrosc 33:796–800
Li YS, Wang Y, Church JS, Garzena F, Zhang ZX, An DQ (2003) Vibrational spectroscopic studies of mesna and dimesna. Spectrochim Acta Part A 59:1791–1798
Wrzosek B, Bukowska J, Kudelski A (2005) Place-exchange reactions of thiols on electrochemically roughened SERS-active silver. Vib Spectrosc 39:257–261
Fujisawa K, Imai S, Suzuki S, Moro-oka Y, Miyashita Y, Yamada Y, Okamoto K (2000) M–S vibrational study in three-coordinate thiolato compounds (NEt4)2 [M(SC6H4-p-X)3] and (NE4t2) [M4(m-SC6H4-p-Cl)6]: M=Cu(I) and Ag(I), X=Cl and Br. J Inorg Biochem 82:229–238
Jeong DH, Jang NH, Suh JS (2000) Photodecomposition of diazanaphthalenes adsorbed on silver colloid surfaces. J Phys Chem B 104:3594–3600
Pergolese B, Muniz-Miranda M, Bigotto A (2005) Surface enhanced Raman scattering investigation of the halide anion effect on the adsorption of 1, 2,3-triazole on silver and gold colloidal nanoparticles. J Phys Chem B 109:9665–9671
Zamarion VM, Timm RA, Araki K, Toma HE (2008) Ultrasensitive SERS nanoprobes for hazardous metal ions based on trimercaptotriazine-modified gold nanoparticles. Inorg Chem 47:2934–2936
Wang L, Li T, Du Y, Chen CG, Li BL, Zhou M, Dong SJ (2010) Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury(II) ions. Biosens Bioelectron 25:2622–2626
Liu ZD, Li YF, Ling J, Huang CZ (2009) A localized surface plasmon resonance light-scattering assay of mercury (II) on the basis of Hg2+-DNA complex induced aggregation of gold nanoparticles. Environ Sci Technol 43:5022–5027
Han DH, Kim Y-R, Oh J-W, Kim TH, Mahajan RK, Kim JS, Kim H (2009) A regenerative electrochemical sensor based on oligonucleotide for the selective determination of mercury (II). Analyst 134:1857–1862
Duan TC, Song XJ, Xu JW, Guo PR, Chen HT, Li HF (2006) Determination of Hg(II) in waters by on-line preconcentration using Cyanex 923 as a sorbent—cold vapor atomic absorption spectrometry. Spectrochim Acta Part B 61:1069–1073
Li J, Mei F, Li W-Y, He X-W, Zhang Y-K (2008) Study on the fluorescence resonance energy transfer between CdTe QDs and butyl-rhodamine B in the presence of CTMAB and its application on the detection of Hg(II). Spectrochim Acta Part A 70:811–817
Chen KH, Wang HW, Kang BS, Chang CY, Wang YL, Lele TP, Ren F, Pearton SJ, Dabiran A, Osinsky A, Chow PP (2008) Low Hg(II) ion concentration electrical detection with AlGaN/GaN high electron mobility transistors. Sens Actuators B 134:386–389
Yang FP, Ma Q, Yu W, Su XG (2011) Naked-eye colorimetric analysis of Hg2+ with bi-color CdTe quantum dots multilayer films. Talanta 84:411–415
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 114 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Wu, L., Chen, Y. et al. Determination of mercury(II) by surface-enhanced Raman scattering spectroscopy based on thiol-functionalized silver nanoparticles. Microchim Acta 177, 341–348 (2012). https://doi.org/10.1007/s00604-012-0777-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0777-6