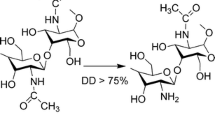
Abstract
Chitosan microspheres were prepared by an emulsion crosslinking method using glutaraldehyde as the cross-linker. Two auxins were dissolved in ethyl benzoate and encapsulated into the microspheres. The best encapsulation efficiency for naphthalene-1-acetic acid and indole-3-acetic acid, respectively, are 68% and 56% and depends on the selection of the appropriate extent of crosslinker, crosslinking time, and the ratio of the oil/water phase. The microspheres were characterized by FTIR spectroscopy. Differential scanning calorimetry was applied to study the thermal stabilities, and scanning electron microscopy to investigate the morphology of the loaded microspheres. In-vitro release studies performed in buffered aqueous methanol at pH 7.4 indicated that the cumulative release rate of the auxins from the particles reaches a maximum (60%) after about 120 h. The release rate in water is higher than the one in methanol. Based on data for the correlation coefficient it is concluded that the drug release is controlled by a diffusion mechanism that follows a super Case-II transport scheme.

In this work, two auxins, e.g., naphthalene-1-acetic acid and indole-3-acetic acid, were encapsulated into chitosan microspheres by an emulsion crosslinking method. Furthermore, the encapsulation efficiency and the in-vitro release were discussed in detail indicating that the drug release was controlled by a diffusion mechanism that followed a super Case-II transport scheme






Similar content being viewed by others
References
Schönherr J, Baur P, Uhlig BA (2000) Rates of cuticular penetration of 1-naphthylacetic acid (NAA) as affected by adjuvants, temperature, humidity and water quality. Plant Growth Regul 31(1):61–74. doi:10.1023/a:1006354732358
Palmer CD, Keller WA (2011) Plant regeneration from petal explants of Hypericum perforatum L. Plant Cell Tissue Organ Cult 105(1). doi:10.1007/s11240-010-9839-9
Lee Y, Lee DE, Lee HS, Kim SK, Lee W, Kim SH, Kim MW (2011) Influence of auxins, cytokinins, and nitrogen on production of rutin from callus and adventitious roots of the white mulberry tree (Morus alba L.). Plant Cell Tissue Organ Cult 105(1):9–19. doi:10.1007/s11240-010-9832-3
Yin C, Wu Q, Zeng H, Xia K, Xu J, Li R (2011) Endogenous auxin is required but supraoptimal for rapid growth of rice (Oryza sativa L.) seminal roots, and auxin inhibition of rice seminal root growth is not caused by ethylene. J Plant Growth Regul 30(1). doi:10.1007/s00344-010-9162-z
Tsatsakis AM, Paritsis KN, Shtilman MI, Shashkova IB, Alegakis AK, Roubelakis-Angelakis KA (1995) 1-Naphthylacetic acid slow release polymeric formulations: auxin type effect in tobacco leaf segments is affected by structure and hydrolysis. Plant Growth Regul 17(2):167–175. doi:10.1007/bf00024177
Pelletier A, Lemire I, Sygusch J, Chornet E, Overend RP (1990) Chitin/chitosan transformation by thermo-mechano-chemical treatment including characterization by enzymatic depolymerization. Biotechnol Bioeng 36(3):310–315. doi:10.1002/bit.260360313
Ghanem A, Skonberg D (2002) Effect of preparation method on the capture and release of biologically active molecules in chitosan gel beads. J Appl Polym Sci 84(2):405–413. doi:10.1002/app.10393
Jameela SR, Lakshmi S, James NR, Jayakrishnan A (2002) Preparation and evaluation of photocrosslinkable chitosan as a drug delivery matrix. J Appl Polym Sci 86(8):1873–1877. doi:10.1002/app.11112
de Oliveira I, Vieira IC (2006) Immobilization procedures for the development of a biosensor for determination of hydroquinone using chitosan and gilo (Solanum gilo). Enzyme Microb Technol 38(3–4):449–456. doi:10.1016/j.enzmictec.2005.06.019
Podzus PE, Daraio ME, Jacobo SE (2009) Chitosan magnetic microspheres for technological applications: Preparation and characterization. Physica B 404(18):2710–2712. doi:10.1016/j.physb.2009.06.093
Chandy T, Sharma CP (1990) Chitosan-as a biomaterial. Artif Cell Blood Substit Biotechnol 18(1):1–24
Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW (2004) A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Contr Release 96(2):285–300. doi:10.1016/j.jconrel.2004.02.002
Jameela SR, Jayakrishnan A (1995) Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle: studies on the in vitro release of mitoxantrone and in vivo degradation of microspheres in rat muscle. Biomaterials 16(10):769–775. doi:10.1016/0142-9612(95)99639-4
Jameela SR, Kumary TV, Lal AV, Jayakrishnan A (1998) Progesterone-loaded chitosan microspheres: a long acting biodegradable controlled delivery system. J Contr Release 52(1–2):17–24. doi:10.1016/s0168-3659(97)00187-9
Jayakrishnan A, Jameela SR (1996) Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 17(5):471–484. doi:10.1016/0142-9612(96)82721-9
Thanoo BC, Sunny MC, Jayakrishnan A (1992) Cross-linked Chitosan Microspheres: preparation and evaluation as a matrix for the controlled release of pharmaceuticals. J Pharm Pharmacol 44(4):283–286. doi:10.1111/j.2042-7158.1992.tb03607.x
Devlieghere F, Vermeulen A, Debevere J (2004) Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol 21(6):703–714. doi:10.1016/j.fm.2004.02.008
Teixeira MA, Paterson WJ, Dunn EJ, Li Q, Hunter BK, Goosen MFA (1990) Assessment of chitosan gels for the controlled release of agrochemicals. Ind Eng Chem Res 29(7):1205–1209. doi:10.1021/ie00103a019
Shtilman M, Tzatzarakis M, Voskanyan P, Tsakiris I, Tsakalof A, Tsatsakis A (2006) Correlation between plant growth regulator release rate and bioactivity for the series of newly synthesized phytoactive polymers. J Plant Growth Regul 25(3):211–218. doi:10.1007/s00344-006-0002-0
Tsatsakis AM, Shtilman MI (1994) Polymeric derivatives of plant growth regulators: synthesis and properties. Plant Growth Regul 14(1):69–77. doi:10.1007/bf00024143
Quinones JP, Garcia YC, Curiel H, Covas CP (2010) Microspheres of chitosan for controlled delivery of brassinosteroids with biological activity as agrochemicals. Carbohydr Polym 80(3):915–921. doi:10.1016/j.carbpol.2010.01.006
Angadi SC, Manjeshwar LS, Aminabhavi TM (2010) Interpenetrating polymer network blend microspheres of chitosan and hydroxyethyl cellulose for controlled release of isoniazid. Int J Biol Macromol 47(2):171–179. doi:10.1016/j.ijbiomac.2010.05.003
Peng HL, Xiong H, Li JH, Xie MY, Liu YZ, Bai CQ, Chen LX (2010) Vanillin cross-linked chitosan microspheres for controlled release of resveratrol. Food Chem 121(1):23–28. doi:10.1016/j.foodchem.2009.11.085
Shao YY, Zhu BQ, Li J, Liu XR, Tan X, Yang XL (2009) Novel chitosan microsphere-templated microcapsules suitable for spontaneous loading of heparin. Mater Sci Eng C Biomimetic Supramol Syst 29(3):936–941. doi:10.1016/j.msec.2008.08.017
Costa P, Sousa Lobo JM (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13(2):123–133. doi:10.1016/s0928-0987(01)00095-1
Dudhani AR, Kosaraju SL (2010) Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym 81(2):243–251. doi:10.1016/j.carbpol.2010.02.026
Kumar P, Singh I (2010) Formulation and characterization of tramadol-loaded IPN microgels of alginate and gelatin: Optimization using response surface methodology. Acta Pharm 60(3):295–310. doi:10.2478/v10007-010-0021-z
Jose S, Prema MT, Chacko AJ, Thomas AC, Souto EB (2011) Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf B Biointerfaces 83(2):277–283. doi:10.1016/j.colsurfb.2010.11.033
Acknowledgement
This work was financially supported by the key Academic Program of the 3rd phase “211 Project”of South China Agricultural University (2009B010100001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, L., Jin, R., Le, X. et al. Chitosan microspheres for controlled delivery of auxins as agrochemicals. Microchim Acta 176, 381–387 (2012). https://doi.org/10.1007/s00604-011-0732-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0732-y