Abstract
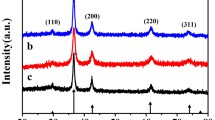
We describe a facile electrochemical route for the synthesis of CuO flower-like microspheres (CuO FMs) by anodic dissolution of bulk Cu in sodium hydroxide solution at room temperature and without heating. Scanning electron microscopy and X-ray diffraction revealed that the CuO FMs are phase-pure monoclinic crystallites and comprised of CuO nanoflakes. The concentration of NaOH has a large effect on the size of the CuO FMs. The possible formation mechanism is discussed. The CuO FMs are electrocatalytically active towards the oxidation of H2O2, and this has resulted in a sensor for H2O2. To our knowledge, this is the simplest way to obtain clean CuO FMs.

A facile electrochemical route, which is carried out at room temperature (25 °C), is introduced for the fast fabrication of CuO flower-like microspheres (CuO FMs). The CuO FMs modified glassy carbon electrode exhibits good electrocatalytic activity towards the oxidation of H2O2.






Similar content being viewed by others
References
Chen LB, Lu N, Xu CM, Yu HC, Wang TH (2009) Electrochemical performance of polycrystalline CuO nanowires as anode material for Li ion batteries. Electrochim Acta 54:4198–4201
Xiang JY, Tu JP, Zhang L, Zhou Y, Wang XL, Shi SJ (2010) Simple synthesis of surface-modified hierarchical copper oxide spheres with needle-like morphology as anode for lithium ion batteries. Electrochim Acta 55:1820–1824
Pan QM, Jin HZ, Wang HB, Yin GP (2007) Flower-like CuO film-electrode for lithium ion batteries and the effect of surface morphology on electrochemical performance. Electrochim Acta 53:951–956
Xiang JY, Tu JP, Huang XH, Yang YZ (2008) A comparison of anodically grown CuO nanotube film and Cu2O film as anodes for lithium ion batteries. J Solid State Electrochem 12:941–945
Korshunov A, Heyrovský M (2009) Electrochemical behavior of copper metal core/oxide shell ultra-fine particles on mercury electrodes in aqueous dispersions. J Electroanal Chem 629:23–29
Ke FS, Huang L, Wei GZ, Xue LJ, Li JT, Zhang B, Chen SR, Fan XY, Sun SG (2009) One-step fabrication of CuO nanoribbons array electrode and its excellent lithium storage performance. Electrochim Acta 54:5825–5829
Manna S, Das K, De SK (2010) Template-free synthesis of mesoporous CuO dandelion structures for optoelectronic applications. ACS Appl Mater Interfaces 2:1536–1542
Xiang JY, Tu JP, Yuan YF, Huang XH, Zhang L, Zhou Y (2009) Improved electrochemical performances of core-shell Cu2O/Cu composite prepared by a simple one-step method. Electrochem Commun 11:262–265
Hu YY, Huang XT, Wang K, Liu JP, Jiang J, Ding RM, Ji XX, Li X (2010) Kirkendall-effect-based growth of dendrite-shaped CuO hollow micro/nanostructures for lithium-ion battery anodes. J Solid State Chem 183:662–667
Sarkar SK, Burla N, Bohannan EW, Switzer JA (2008) Inducing enantioselectivity in electrodeposited CuO films by chiral etching. Electrochim Acta 53:6191–6195
Satheesh Babu TGS, Ramachandran T (2010) Development of highly sensitive non-enzymatic sensor for the selective determination of glucose and fabrication of a working model. Electrochim Acta 55:1612–1618
Liu Y, Chu Y, Zhuo YJ, Li MY, Li LL, Dong LH (2007) Anion-controlled construction of CuO honeycombs and flowerlike assemblies on copper foils. Cryst Growth Des 7:467–470
Vaseem M, Umar A, Kim SH, Hahn YB (2008) Low-temperature synthesis of flower-shaped CuO nanostructures by solution process: formation mechanism and structural properties. J Phys Chem C 112:5729–5735
Xu YY, Chen DR, Jiao XL (2005) Fabrication of CuO pricky microspheres with tunable size by a simple solution route. J Phys Chem B 109:13561–13566
Lu CH, Qi LM, Yang JH, Zhang DY, Wu NZ, Ma JM (2004) Simple template-free solution route for the controlled synthesis of Cu(OH)2 and CuO nanostructures. J Phys Chem B 108:17825–17831
Zhang XJ, Wang GF, Liu XW, Wu JJ, Li M, Gu J, Liu H, Fang B (2008) Different CuO nanostructures: synthesis, characterization, and applications for glucose sensors. J Phys Chem C 112:16845–16849
Ni YH, Li H, Jin LN, Hong JM (2009) Synthesis of 1D Cu(OH)2 nanowires and transition to 3D CuO microstructures under ultrasonic irradiation, and their electrochemical property. Cryst Growth Des 9:3868–3873
Xu HL, Wang WZ, Zhu W, Zhou L, Ruan ML (2007) Hierarchical-oriented attachment: from one-dimensional Cu(OH)2 nanowires to two-dimensional CuO nanoleaves. Cryst Growth Des 7:2720–2724
Jiang XC, Herricks T, Xia YN (2002) CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett 2:1333–1338
Murray BJ, Li Q, Newberg JT, Menke EJ, Hemminger JC, Penner RM (2005) Shape- and size-selective electrochemical synthesis of dispersed silver(I) oxide colloids. Nano Lett 5:2319–2324
Chen X, Chen S, Huang W, Zheng JF, Li ZL (2009) Facile preparation of Bi nanoparticles by novel cathodic dispersion of bulk bismuth electrodes. Electrochim Acta 54:7370–7373
Qiu HJ, Lu L, Xue LY, Huang XR (2010) Facile electrochemical preparation of three-dimensional porous Cu films by potential perturbation. Electrochim Acta 55:6081–6087
Qiu HJ, Lu L, Huang XR, Qu YB (2010) Facile preparation of Cu2O microcrystals with morphologies of octahedron, half circular and rectangular plates by anodic dissolution of bulk Cu in alkaline aqueous solutions. Electrochim Acta 56:291–296
Caballero-Briones F, Palacios-Padrós A, Calzadilla O, Sanz F (2010) Evidence and analysis of parallel growth mechanisms in Cu2O films prepared by Cu anodization. Electrochim Acta 55:4353–4358
Burke LD, Bruton GM, Collins JA (1998) The redox properties of active sites and the importance of the latter in electrocatalysis at copper in base. Electrochim Acta 44:1467–1479
Ribotta SB, La orgia LF, Gassa LM, Folquer ME (2008) Characterization of anodic films formed on copper in 0.1 M borax solution. J Electroanal Chem 624:262–268
Kunze J, Maurice V, Klein LH, Strehblow HH, Marcus P (2004) In situ STM study of the duplex passive films formed on Cu(1 1 1) and Cu(0 0 1) in 0.1 M NaOH. Corr Sci 46:245–264
Tatsuma T, Ogawa T, Sato R, Oyama N (2001) Peroxidase-incorporated sulfonated polyaniline–polycation complexes for electrochemical sensing of H2O2. J Electroanal Chem 501:180–185
Mendes RK, Carvalhal RF, Kubota LT (2008) Effects of different self-assembled monolayers on enzyme immobilization procedures in peroxidase-based biosensor development. J Electroanal Chem 612:164–172
Qiu HJ, Xue LY, Ji GL, Zhou GP, Huang XR, Qu YB, Gao PJ (2009) Enzyme-modified nanoporous gold-based electrochemical biosensors. Biosens Bioelectron 24:3014–3018
Wei H, Chen CG, Han BY, Wang EK (2008) Enzyme colorimetric assay using unmodified silver nanoparticles. Anal Chem 80:7051–7055
Zhang L, Ni YH, Li H (2010) Addition of porous cuprous oxide to a Nafion film strongly improves the performance of a nonenzymatic glucose sensor. Microchim Acta 171:103–108
Wang Q, Zheng JB (2010) Electrodeposition of silver nanoparticles on a zinc oxide film: improvement of amperometric sensing sensitivity and stability for hydrogen peroxide determination. Microchim Acta 169:361–365
Ping JF, Ru SP, Fan K, Wu J, Ying YB (2010) Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim Acta 171:117–123
Xiang JY, Tu JP, Zhang L, Zhou Y, Wang XL, Shi SJ (2010) Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J Power Sources 195:313–319
Jia WZ, Guo M, Zheng Z, Yu T, Wang Y, Rodriguez EG, Lei Y (2008) Vertically aligned CuO nanowires based electrode for amperometric detection of hydrogen peroxide. Electroanalysis 19:2153–2157
Miao XM, Yuan R, Chai YQ, Shi YT, Yuan YY (2008) Direct electrocatalytic reduction of hydrogen peroxide based on Nafion and copper oxide nanoparticles modified Pt electrode. J Electroanal Chem 612:157–163
Acknowledgments
The authors gratefully acknowledge the financial support from State Key Laboratory of Microbial Technology of China, the Provincial Natural Science Foundation of Shandong (Y2008B13), the National Natural Science Foundation of China (20973103) and the National Basic Research Program of China (2011CB707400).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Huang, X. Room temperature electrochemical synthesis of CuO flower-like microspheres and their electrooxidative activity towards hydrogen peroxide. Microchim Acta 175, 151–157 (2011). https://doi.org/10.1007/s00604-011-0663-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0663-7