Abstract
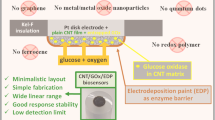
We describe a microbial sensor based on Pseudomonas fluorescens cells that was prepared by modifying graphite electrodes with chitosan and carbon nanotubes. Chronoamperometry was performed at +0.3 V in the presence of hexacyanoferrate as a mediator and revealed a good response to glucose which is linear in the 1.0 to 5.0 mM concentration range. Linearity was defined by the equation of y = 102.120x−13.279 (R 2 = 0.998) (y shows current density as nA.cm−2 and x shows glucose concentration in mM). The effect of the CNTs on the response was compared to that of electrodes made without CNTs.

A mediated microbial sensor that was prepared by modifying graphite electrodes with chitosan and carbon nanotube and Pseudomonas fluorescens cells has been described. As well as some parameters (pH, mediator and cell amount etc), the effect of CNTs on the response was compared to that of electrodes made without CNTs.





Similar content being viewed by others
References
Wu BY, Hou SH, Yu M, Qin X, Li S, Chen Q (2009) Layer-by-layer assemblies of chitosan/multi-wall carbon nanotubes and glucose oxidase for amperometric glucose biosensor applications. Mat Sci Eng C 29:346
Lin Y, Taylor S, Li H, Fernando KAS, Qu L, Wang W, Gu L, Zhou B, Sun YP (2004) Advances toward bioapplications of carbon nanotubes. J Mater Chem 14:527
Rahman MM, Shiddiky MJ, Rahman MA, Shim YB (2009) A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multiwall carbon nanotube composite film. Anal Biochem 384:159
Jeykumari DRS, Narayanan SS (2009) Functionalized carbon nanotubes-bienzyme biocomposite for amperometric sensing. Carbon 47:957
Odaci D, Telefoncu A, Timur S (2008) Pyranose oxidase biosensor based on carbon nanotube (CNT)-modified carbon paste electrodes. Sensors Actuat B Chem 132:159
Lin J, He C, Zhang L, Zhang S (2009) Sensitive amperometric immunosensor for α-fetoprotein based on carbon nanotube/gold nanoparticle doped chitosan film. Anal Biochem 384:130
Yang M, Kostov Y, Rasooly A (2008) Carbon nanotubes based optical immunodetection of Staphylococcal Enterotoxin B (SEB) in food. Int J Food Microbiol 127:78
Erdem A (2007) Nanomaterial-based electrochemical DNA sensing strategies. Talanta 74:318
Odaci D, Timur S, Telefoncu A (2008) Bacterial sensors based on chitosan matrices. Sens Actuat B Chem 134:89
Kirgoz UA, Timur S, Odaci D, Perez B, Alegret S, Merkoçi A (2007) Carbon nanotube composite as novel platform for microbial biosensor. Electroanal 19:893
Timur S, Anik U, Odaci D, Gorton L (2007) Development of a microbial biosensor based on carbon nanotube (CNT) modified electrodes. Electrochem Commun 9:1810
Qiu JD, Deng MQ, Liang RP, Xiong M (2008) Ferrocene-modified multiwalled carbon nanotubes as building block for construction of reagentless enzyme-based biosensors. Sensor Actuat B Chem 135:181
Wang J, Liu G, Jan MR, Zhu Q (2003) Electrochemical detection of DNA hybridization based on carbon-nanotubes loaded with CdS tags. Electrochem Commun 5:1000
Li Q, Kinloch IA, Windle AH (2005) Discrete dispersion of single-walled carbon nanotubes. Chem Commun 26:3283
Jin HJ, Choi HJ, Yoon SH, Myung SJ, Shim SE (2005) Carbon nanotube-adsorbed polystyrene and poly(methyl methacrylate) microspheres. Chem Mater 17:4034
Wu K, Sun Y, Hu S (2003) Development of an amperometric indole-3-acetic acid sensor based on carbon nanotubes film coated glassy carbon electrode. Sensors Actuat B Chem 96:658
Rouse JH, Lillehei PT, Sanderson J, Siochi EJ (2004) Polymer/single-walled carbon nanotube films assembled via donor−acceptor ınteractions and their use as scaffolds for silica deposition. Chem Mater 16:3904
Tkac J, Ruzgas T (2006) Dispersion of single walled carbon nanotubes. Comparison of different dispersing strategies for preparation of modified electrodes toward hydrogen peroxide detection. Electrochem Commun 8:899
Liu JT, Chen X, Xin JH (2005) Decoration of carbon nanotubes with chitosan. Carbon 43:3178
Gonchar M, Maidan M, Korpan Y, Sibirny V, Kotylak Z, Sibirny A (2002) Metabolically engineered methylotrophic yeast cells and enzymes as sensor biorecognition elements. FEMS Yeast Res 2:307
D’Souza SF (2001) Microbial biosensors. Biosens Bioelectron 16:337
Pasco N, Baronian K, Jeffries C, Webber J, Hay J (2004) MICREDOX®-development of a ferricyanide-mediated rapid biochemical oxygen demand method using an immobilised Proteus vulgaris biocomponent. Biosens Bioelectron 20:524
Kirgoz UA, Odaci D, Timur S, Merkoçi A, Pazarlıoğlu N, Telefoncu A, Alegret S (2006) Graphite epoxy composite electrodes modified with bacterial cells. Bioelectrochemistry 69:128
Nakanishi K, Ikebukuro K, Karube I (1996) Determination of cyanide using a microbial sensor. Appl Biochem Biotech 60:97
Valdman E, Battaglini F, Leite SGF, Valdman B (2004) Naphthalene detection by a bioluminescence sensor applied to wastewater samples. Sensors Actuat B Chem 103:7
Odaci D, Sezginturk MK, Timur S, Pazarlioglu N, Pilloton R, Dinckaya E, Telefoncu A (2009) Pseudomonas putida based amperometric biosensors for 2,4-D detection. Prep Biochem Biotech 39:11
Tkac J, Vostiar I, Gemeiner P, Sturdik E (2002) Monitoring of ethanol during fermentation using a microbial biosensor with enhanced selectivity. Biosens Bioelectron 56:127
Reshetilov AN, Trotsenko JA, Morozova NO, Iliasov PV, Ashin VV (2001) Characteristics of Gluconobacter oxydans B-1280 and Pichia methanolica MN4 cell based biosensors for detection of ethanol. Process Biochem 36:1015
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25:464
Timur S, Odaci D, Dinçer A, Zihnioglu F, Telefoncu A (2008) Biosensing approach for glutathione detection using glutathione reductase and sulfhydryl oxidase bienzymatic system. Talanta 74:1492
Balasubramanian K, Burghard M (2006) Biosensors based on carbon nanotubes. Anal Bioanal Chem 385:452
Wang J (2004) Carbon-nanotube based electrochemical biosensors: a review. Electroanal 17:7
Ghica ME, Brett CMA (2010) The influence of carbon nanotubes and polyazine redox mediators on the performance of amperometric enzyme biosensors. Microchim Acta 170:257
Yang Q, Qu Y, Bo Y, Wen Y, Huang S (2010) Biosensor for atrazin based on aligned carbon nanotubes modified with glucose oxidase. Microchim Acta 168:197
Chaubey A, Malhotra BD (2002) Mediated biosensors. Biosens Bioelectron 17:441
Lei Y, Chen W, Mulchandanib A (2006) Microbial biosensors. Anal Chim Acta 568:200
Yoshida N, Yano K, Morita T, McNiven SJ, Nakamura H, Karube I (2000) A mediator-type biosensor as a new approach to biochemical oxygen demand estimation. Analyst 125:2280
Nakamura H, Suzuki K, Ishikuro H, Kinoshita S, Koizumi R, Okuma S, Gotoh M, Karube I (2007) A new BOD estimation method employing a double-mediator system by ferricyanide and menadione using the eukaryote Saccharomyces cerevisiae. Talanta 72:210
Pasco N, Hay J, Scott A, Webber J (2005) Redox coupling to microbial respiration: an evaluation of secondary mediators as binary mixtures with ferricyanide. Aust J Chem 58:288
Rivas GA, Rubianes MD, Rodriguez MC, Ferreyra NF, Luque GL, Pedano ML, Miscoria SA, Parrado C (2007) Carbon nanotubes for electrochemical biosensing. Talanta 74:291
Zhang M, Gorski W (2005) Electrochemical sensing platform based on the carbon nanotubes/redox mediators-biopolymer system. J Am Chem Soc 127:2058
Liu S, Yu J, Ju H (2003) Renewable phenol biosensor based on a tyrosinase-colloidal gold modified carbon paste electrode. Electroanal Chem 540:61
Su L, Jia W, Hou C, Lei Y (2011) Microbial biosensors: a review. Biosens Bioelectron 26:1788
Acknowledgement
Ege University Research Foundation (project number: 2010 FEN 001) is acknowledged for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Demirkol, D.O., Timur, S. Chitosan matrices modified with carbon nanotubes for use in mediated microbial biosensing. Microchim Acta 173, 537–542 (2011). https://doi.org/10.1007/s00604-011-0596-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0596-1