Abstract
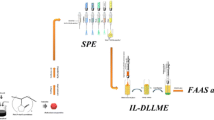
Activated carbon was chemically modified with ethyl-3-(2-aminoethylamino)-2-chlorobut-2-enoate to obtain a material for selective solid-phase extraction of trace Au(III), Pd(II) and Pt(IV) prior to their determination by inductively coupled plasma atomic emission spectrometry. Experimental conditions such as effects of pH, shaking time, sample flow rate and volume, elution and interfering ions were studied. The ions Au(III), Pd(II) and Pt(IV) can be quantitatively adsorbed on the new sorbent from solution of pH 1. The adsorbed ions were then eluted with 0.1 mol L−1 hydrochloric acid and containing 4% thiourea. Many common ions do not interfere. The adsorption capacity of the material is 305, 92, and 126 mg g−1 for Au(III), Pd(II) and Pt(IV), respectively, and the detection limits are 5, 11 and 9 ng mL−1. The relative standard deviation is less than 3.0% (n = 8) under optimum conditions. The method was validated by analyzing two certified reference materials and successfully applied to the preconcentration and determination of these ions in actual samples with satisfactory results.

Activated carbon was chemically modified with ethyl-3-(2-aminoethylamino)-2-chlorobut-2-enoate to obtain a material for selective solid-phase extraction of trace Au(III), Pd(II) and Pt(IV) prior to their determination by inductively coupled plasma atomic emission spectrometry. Parameters affecting solid-phase extraction were systematically studied. This new adsorbent exhibited good characteristics for separation and preconcentration of Au(III), Pd(II) and Pt(IV) in aqueous solution, such as excellent selectivity, fast adsorption equilibrium, high tolerance limits of potentially interfering ions, high enrichment factor and low costs. It also shows relatively high adsorption capacity when compared to several other adsorbents. In addition, the synthetic method of the adsorbent was very simple.





Similar content being viewed by others
References
Liu P, Liu GF, Chen DL, Cheng SY, Tang N (2009) Adsorption properties of Ag(I), Au(III), Pd(II) and Pt(IV) ions on commercial 717 anion-exchange resin. Trans Nonferr Met Soc China 19:1509
Masllorens J, Roglans A, Anticó E, Fontàs C (2006) New applications of azamacrocyclic ligands in ion recognition, transport and preconcentration of palladium. Anal Chim Acta 560:77
Maruyama T, Matsushita H, Shimada Y, Kamata I, Hanaki M, Sonokawa S, Kamiya N, Goto M (2007) Proteins and Protein-Rich Biomass as Environmentally Friendly Adsorbents Selective for Precious Metal Ions. Environ Sci Technol 41:1359
Fujiwara K, Ramesh A, Maki T, Hasegawa H, Ueda K (2007) Adsorption of platinum(IV), palladium(II) and gold(III) from aqueous solutions onto L-lysine modified crosslinked chitosan resin. J Hazard Mater 146:39
Leśniewska BA, Godlewska-Żyłkiewicz B, Ruszczyńska A, Bulska E, Hulanicki A (2006) Elimination of interferences in determination of platinum and palladium in environmental samples by inductively coupled plasma mass spectrometry. Anal Chim Acta 564:236
Ravindra K, Bencs L, Van Grieken R (2004) Platinum group elements in the environment and their health risk. Sci Total Environ 318:1
Gómez B, Palacios MA, Gómez M, Sanchez JL, Morrison G, Rauch S, McLeod C, Ma R, Caroli S, Alimonti A, Petrucci F, Bocca B, Schramel P, Zischka M, Petterson C, Wass U (2002) Levels and risk assessment for humans and ecosystems of platinum-group elements in the airborne particles and road dust of some European cities. Sci Total Environ 299:1
Silva MAM, Frescura VLA, Curtius AJ (2001) Determination of noble metals in biological samples by electrothermal vaporization inductively coupled plasma mass spectrometry, following cloud point extraction. Spectrochim Acta B 56:1941
Dai XX, Koeberl C, Fröschl H (2001) Determination of platinum group elements in impact breccias using neutron activation analysis and ultrasonic nebulization inductively coupled plasma mass spectrometry after anion exchange preconcentration. Anal Chim Acta 436:79
Zhang SM, Pu QS, Liu P, Sun QY, Su ZX (2002) Synthesis of amidinothioureido-silica gel and its application to flame atomic absorption spectrometric determination of silver, gold and palladium with on-line preconcentration and separation. Anal Chim Acta 452:223
Soylak M, Erdogan ND (2006) Copper(II)–rubeanic acid coprecipitation system for separation–preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. J Hazard Mater B 137:1035
Tuzen M, Saygi KO, Soylak M (2008) Novel solid phase extraction procedure for gold(III) on Dowex M 4195 prior to its flame atomic absorption spectrometric determination. J Hazard Mater 156:591
Tokahoğlu Ş, Yılmaz V, Kartal Ş, Delibaş A, Soykan C (2009) Solid phase extraction of Pd(II) on a newly synthesized chelating resin prior to determination by flame atomic absorption spectrometry. Microchim Acta 165:347
Tong SS, Jia Q, Song NZ, Zhou WH, Duan TC, Bao CL (2010) Determination of gold(III) and palladium(II) in mine samples by cloud point extraction preconcentration coupled with flame atomic absorption spectrometry. Microchim Acta. doi:10.1007/s00604-010-0466-2
Mohammadi SZ, Afzali D, Taher MA, Baghelani YM (2010) Determination of trace amounts of palladium by flame atomic absorption spectrometry after ligandless-dispersive liquid-liquid microextraction. Microchim Acta 168:123
Chang XJ, Su ZX, Yang D, Gong BL, Pu QS, Li SK (1997) Synthesis and efficiency of a spherical macroporous epoxy-imidazole complexing resin for preconcentrating trace noble metal ions. Anal Chim Acta 354:143
Hang CZ, Hu B, Jiang ZC, Zhang N (2007) Simultaneous on-line preconcentration and determination of trace metals in environmental samples using a modified nanometer-sized alumina packed micro-column by flow injection combined with ICP-OES. Talanta 71:1239
Nakajima J, Ohno M, Chikama K, Seki T, Oguma K (2009) Determination of traces of palladium in stream sediment and auto catalyst by FI-ICP-OES using on-line separation and preconcentration with QuadraSil TA. Talanta 79:1050
Jankowski K, Jackowska A, Łukasiak P (2005) Determination of precious metals in geological samples by continuous powder introduction microwave induced plasma atomic emission spectrometry after preconcentration on activated carbon. Anal Chim Acta 540:197
Barefoot RR (1998) Determination of the precious metals in geological materials by inductively coupled plasma mass spectrometry. J Anal At Spectrom 13:1077
Rauch S, Motelica-Heino M, Morrison GM, Donard OFX (2000) Critical assessment of platinum group element determination in road and urban river sediments using ultrasonic nebulisation and high resolution ICP-MS. J Anal At Spectrom 15:329
Schierl R (2000) Environmental monitoring of platinum in air and urine. Microchem J 67:245
Wu YW, Jiang ZC, Hu B, Duan JK (2004) Electrothermal vaporization inductively coupled plasma atomic emission spectrometry determination of gold, palladium, and platinum using chelating resin YPA4 as both extractant and chemical modifier. Talanta 63:585
Vaezzadeh M, Shemirani F, Majidi B (2010) Microextraction technique based on ionic liquid for preconcentration and determination of palladium in food additive, sea water, tea and biological samples. Food Chem Toxicol 48:1455
Ghaedi M, Shabani R, Shokrollahi A, Montazerozohori M, Sahraiean A, Soylak M (2009) Preconcentration and separation of trace amount of copper(II) on N1, N2-bis(4-fluorobenzylidene)ethane-1, 2-diamine loaded on Sepabeads SP70. J Hazard Mater 170:169
Tu ZF, He Q, Chang XJ, Hu Z, Gao R, Zhang LN, Li ZH (2009) 1-(2-Formamidoethyl)-3-phenylurea functionalized activated carbon for selective solid-phase extraction and preconcentration of metal ions. Anal Chim Acta 649:252
He Q, Hu Z, Jiang Y, Chang XJ, Tu ZF, Zhang LN (2010) Preconcentration of Cu(II), Fe(III) and Pb(II) with 2-((2-aminoethylamino)methyl)phenol-functionalized activated carbon followed by ICP-OES determination. J Hazard Mater 175:710
Tian H, Chang XJ, Hu Z, Yang K, He Q, Zhang LN, Tu ZF (2010) Activated carbon modified with 4-(8-hydroxyquinoline-azo) benzamidine for selective solid-phase extraction and preconcentration of trace lead from environmental samples. Microchim Acta 171:225
Garcia L, Torrent A, Anticó E, Fontàs C, Roglans A (2008) Selective Pd(II) and Pt(IV) sorption using novel polymers containing azamacrocycle functional groups. React Funct Polym 68:1088
Birinci E, Gülfen M, Aydın AO (2009) Separation and recovery of palladium(II) from base metal ions by melamine–formaldehyde–thiourea (MFT) chelating resin. Hydrometallurgy 95:15
Laxen DPH, Harrison RM (1981) Cleaning methods for polythene containers prior to the determination of trace metals in fresh water samples. Anal Chem 53:345
Su ZX, Pu QS, Luo XY, Chang XJ, Zhan GY, Ren FZ (1995) Application of a macroporous resin containing imidazoline groups to preconcentration and separation of gold, platinum and palladium prior to ICP-AES determination. Talanta 42:1127
Schultz AG, Hagmann WK (1978) Synthesis of indole-2-carboxylic esters. J Org Chem 43:3391
Tang HT (1992) Organic compound spectra determination. Publishing House of Beijing University, Beijing
Dong QN (1979) IR spectrum method. Publishing House of the Chemical Industry, Beijing
Ramesh A, Hasegawa H, Sugimoto W, Maki T, Ueda K (2008) Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Bioresour Technol 99:3801
Liu R, Liang P (2007) Determination of gold by nanometer titanium dioxide immobilized on silica gel packed microcolumn and flame atomic absorption spectrometry in geological and water samples. Anal Chim Acta 604:114
Uheida A, Iglesias M, Fontas C, Hidalgo M, Salvado V, Zhang Y, Muhammed M (2006) Sorption of palladium(II), rhodium(III), and platinum(IV) on Fe3O4 nanoparticles. J Colloid Interface Sci 301:402
Takeda K, Kawakami F, Sasaki M (1984) Ion-exchange equilibrium behavior of complex ions in Fe3+–Cl− and UO 2+2 –Cl− systems. Nihonkagakukaishi 2:1138
Senturk HB, Gundogdu A, Bulut VN, Duran C, Soylak M, Elci L, Tufekci M (2007) Separation and enrichment of gold(III) from environmental samples prior to its flame atomic absorption spectrometric determination. J Hazard Mater 149:317
Park C, Chung JS, Cha KW (2000) Separation and preconcentration method for palladium, platinum and gold from some heavy metals using Amberlite IRC 718 chelating Resin. Bull Korean Chem Soc 21:121
Pu Q, Su Z, Hu Z, Chang X, Yang M (1998) 2-Mercaptobenzothiazole-bonded silica gel as selective adsorbent for preconcentration of gold, platinum and palladium prior to their simultaneous inductively coupled plasma optical emission spectrometric determination. J Anal At Spectrom 13:249
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, Z., Lu, S., Chang, X. et al. Selective solid-phase extraction and separation of trace gold, palladium and platinum using activated carbon modified with ethyl-3-(2-aminoethylamino)-2-chlorobut-2-enoate. Microchim Acta 173, 231–239 (2011). https://doi.org/10.1007/s00604-011-0552-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0552-0