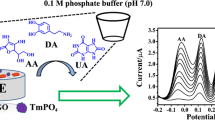
Abstract
Nanocomposites composed of cuprous oxide (Cu2O) and graphene were synthesized via reduction of copper(II) in ethylene glycol. This material possesses the specific features of both Cu2O and graphene. Its morphology was characterized by X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and X-ray photoelectron spectroscopy. Cyclic voltammetry was used to evaluate the electrochemical response of a glass carbon electrode (GCE) modified with the nanocomposite towards dopamine (DA). Compared to the bare GCE, the Cu2O nanoparticles modified electrode and the graphene modified electrode, the nanocomposites modified electrode displays high electrocatalytic activity in giving an oxidation peak current that is proportional to the concentration of DA in the range from 0.1 to 10 μM,with a detection limit of 10 nM (S/N = 3). The modified electrode shows excellent selectivity and sensitivity even in the presence of high concentration of uric acid and can be applied to determine DA in real samples with satisfactory results.

Cu2O/Graphene nanocomposites were successfully prepared, Cu2O particles were uniformly distributed on transparent graphene and no particles scattered out of the supports. Electrochemical experiment results indicate that the nanocomposites modified electrode displays a wide linear region, excellent selectivity and sensitivity to DA.





Similar content being viewed by others
References
Wen XL, Jia YH, Liu ZL (1999) Micellar effects on the electrochemistry of dopamine and its selective detection in the presence of ascorbic acid. Talanta 50:1027
Wu K, Fei J, Hu S (2003) Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal Biochem 318:100
Mo JW, Ogorevc B (2001) Simultaneous measurement of dopamine and ascorbate at their physiological levels using voltammetric microprobe based on overoxidized poly (1, 2-phenylenediamine)-coated carbon fiber. Anal Chem 73:1196
Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain Michael. Anal Chem 60:769A
Clszewski A, Milczarek G (1999) Polyeugenol-modified platinum electrode for selective detection of dopamine in the presence of ascorbic acid. Anal Chem 71:1055
Zhang YZ, Cai YJ, Su S (2006) Determination of dopamine in the presence of ascorbic acid by poly (styrene sulfonic acid) sodium salt/single-wall carbon nanotube flim modified glassy carbon electrode. Anal Biochem 350:285
Shams E, Babaei A, Taheri AR, Kooshki M (2009) Voltammetric determination of dopamine at a zirconium phosphated silica gel modified carbon paste electrode. Bioelectrochemistry 75:83
Wang Y, Li YM, Tang LH, Lu J, Li JH (2009) Application of graphene-modified electrode for selective detection of dopamine. Electrochem Commun 11:889
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666
Alwarappan S, Erdem A, Liu C, Li CZ (2009) Probing the electrochemical properties of graphene nanosheets for biosensing applications. J Phys Chem C 113:8853
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282
Gilje S, Han S, Wang MS, Wang KL, Kaner RB (2007) A chemical route to graphene for device applications. Nano Lett 7:3394
Wu JB, Becerril HA, Bao ZA, Liu ZF, Chen YS, Peter P (2008) Organic solar cells with solution-processed graphene transparent electrode. Appl Phys Lett 92:263302
Wu H, Wang J, Kang XH, Wang CM, Wang DH, Liu J, Aksay IA, Lin YH (2009) Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 80:403
Shan CS, Yang HF, Song JF, Han DX, Ivaska A, Niu L (2009) Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem 81:2378
Zhou KF, Zhu YF, Yang XL, Luo J, Li CZ, Luan SR (2010) A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochim Acta 55:3055
Lin WJ, Liao CS, Jhang JH, Tsai YC (2009) Graphene modified basal and edge plane pyrolytic graphite electrodes for electrocatalytic oxidation of hydrogen peroxide and b-nicotinamide adenine dinucleotide. Electrochem Commun 11:2153
Zhou M, Zhai Y, Dong S (2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem 81:5603
Wang Y, Wan Y, Zhang D (2010) Reduced graphene sheets modified glassy carbon electrode for electrocatalytic oxidation of hydrazine in alkaline media. Electrochem Commun 12:187–190
Wang C, Zhang L, Guo ZH, Xu JG, Wang HY, Zhai KF, Zhuo X (2010) A novel hydrazine electrochemical sensor based on the high specific surface area grapheme. Microchim Acta 169:1
Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, Kim JS, Kim H (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron 25:2366
Zhang HG, Zhu QS, Zhang Y, Wang Y, Zhao L, Yu B (2007) One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv Funct Mater 17:2766
Gou LF, Murphy CJ (2003) Solution-Phase Synthesis of Cu2O Nanocubes. Nano Lett 3:231–234
Orel ZC, Anzlovar A, Drazic G, Zigon M (2007) Cuprous oxide nanowires prepared by an additive-free polyol process. Cryst Growth Des 7:453
Zhang XJ, Wang GF, Wang Q, Zhao LJ, Wang M, Fang B (2009) Cupreous oxide nanobelts as detector for determination of l-Tyrosine. Mater Sci Eng, B 156:6
Zhang XJ, Wang GF, Gu AX, Wu HQ, Fang B (2008) Preparation of porous Cu2O octahedron and its application as L-Tyrosine sensors. Solid State Commun 148:525
Zhang XJ, Wang GF, Zhang W, Wei Y, Fang B (2009) Fixure-reduce method for the synthesis of Cu2O/MWCNTs nanocomposites and its application as enzyme-free glucose sensor. Biosens Bioelectron 24:3395
Shishiyanu ST, Shishiyanu TS, Lupan OI (2006) Novel NO2 gas sensor based on cuprous oxide thin films. Sensors Actuators B 113:468
Rajamathi M, Seshadri R (2002) Oxide and chalcogenide nanoparticles from hydrothermal/solvothermal reactions. Curr Opin Solid State Mater Sci 6:337
Li YJ, Gao W, Ci LJ, Wang CM, Ajayan PM (2010) Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 48:1124
Liu S, Wang JQ, Zeng J, Ou JF, Li ZP, Liu XH, Yang SR (2010) “Green” electrochemical synthesis of Pt/graphene sheet nanocomposite filmand its electrocatalytic property. J Power Sources 195:4628
Chien CC, Jeng KT (2006) Effective preparation of carbon nanotube supported Pt–Ru electrocatalysts. Mater Chem Phys 99:80
Niu JJ, Wang JN (2008) Activated carbon nanotubes-supported catalyst in fuel cells. Electrochim Acta 53:8058
Xu C, Wang X, Zhu JW (2008) Graphene-metal particle nanocomposites. J Phys Chem C 112:19841
Wu JL, Shen XP, Jiang L, Wang K, Chen KM (2010) Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites. Appl Surf Sci 256:2826
Xing YC (2004) Synthesis and electrochemical characterization of uniformly-dispersed high loading Pt nanoparticles on sonochemically-treated carbon nanotubes. J Phys Chem B 108:19255
Xu C, Wang X, Yang LC, Wu YP (2009) Fabrication of a graphene–cuprous oxide composite. J Solid State Chem 182:2486
Kuo CH, Huang MH (2008) Fabrication of truncated rhombic dodecahedral Cu2O Nanocages and Nanoframes by particle aggregation and acidic etching. J Am Chem Soc 130:12815
Angeles GA, Lopez BP, Pardave MP, Silva MTR, Alegret S, Merkoci A (2008) Enhanced host–guest electrochemical recognition of dopamine using cyclodextrin in the presence of carbon nanotubes. Carbon 46:898
Jin GP, Lin XQ, Gong JM (2004) Novel choline and acetylcholine modified glassy carbon electrodes for simultaneous determination of dopamine, serotonin and ascorbic acid. J Electroanal Chem 569:135
Ke NJ, Lu SS, Cheng SH (2006) A strategy for the determination of dopamine at a bare glassy carbon electrode: p-Phenylenediamine as a nucleophile. Electrochem Comm 8:1514
Kang G, Lin X (2006) RNA modified electrodes for simultaneous determination of dopamine and uric acid in the presence of high amounts of ascorbic acid. Electroanalysis 18:2458–2466
Wang ZH, Liu J, Liang QL, Wang YM, Luo GA (2002) Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst 127:653
Gelbert MB, Curran DJ (1986) Alternating current voltammetry of dopamine and ascorbic acid at carbon paste and stearic acid modified carbon paste electrodes. Anal Chem 58:1028
Wang P, Li YX, Huang X, Wang L (2007) Fabrication of layer-by-layer modified multilayer films containing choline and gold nanoparticles and its sensing application for electrochemical determination of dopamine and uric acid. Talanta 73:431
Ge PY, Du Y, Xu JJ, Chen HY (2009) Selective detection of dopamine based on the unique property of gold nanofilm. J Electroanal Chem 633:182
Kumar SA, Tang CF, Chen SM (2008) Poly (4-amino-1-1′-azobenzene-3, 4′-disulfonic acid) coated electrode for selective detection of dopamine from its interferences. Talanta 74:860
Sun D, Xie XF, Zhang HJ (2010) Surface effects of mesoporous silica modified electrode and application inelectrochemical detection of dopamine. Colloids Surf, B 75:88
He SY, Ren BY, Liu XX, Tong Z (2010) Reversible electrogelation in poly (acrylic acid) aqueous solutions triggered by redox reactions of counterionsa. Macromol Chem Phys doi: 10.1002/macp.201000429
Acknowledgement
This work is financially supported by the National Nature Science Foundation of China (NO.20775030).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
(DOC 1262 kb)
Rights and permissions
About this article
Cite this article
Zhang, F., Li, Y., Gu, Ye. et al. One-pot solvothermal synthesis of a Cu2O/Graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173, 103–109 (2011). https://doi.org/10.1007/s00604-010-0535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0535-6