Abstract
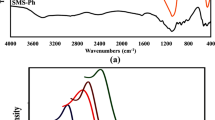
A novel, single-step route has been developed for the synthesis of solid phase adsorbent silica modified with xylenol orange. The addition of cationic surfactant cetyl tri-methylammonium bromide during the synthesis of the adsorbent supports the formation of a stable coating of xylenol orange on silica. The adsorbent showed no signs of degradation in contact with organic solvents and with solutions of varying pH between 1 and 9. This adsorbent has been used for separation and pre-concentration of uranium from hydro-geochemical samples with high calcium content and from sea water. Quantitative sorption of uranium was observed above pH 3 and complete desorption can be achieved using 0.2 M sodium pyrophosphate solution. The uranium content in the extract was determined by laser fluorimetric technique. The equilibration time is 30 min. The sorption capacity of the adsorbent for uranium is 10 mg g−1. An enrichment factor of 50 was obtained by this procedure taking 500 mL of sample solution. Uranium concentrations down to 0.05 ng mL−1 can be determined after pre-concentration using this method. The relative standard deviation at an 0.1 ng mL−1 level is ±15%.



Similar content being viewed by others
References
Puri BK, Salake M, Usami S (1987) Selective Pre-concentration of iron in beverages and water samples using 2, 4, 6, tri- pyridinyl 1, 3, 5 triazine tetra phenyl borate naphthalene. Anal Chem 59:1850
Obala H, Keratini H, Nakayama C (1993) Automated determination of iron in sea water by chelating resin concentration and chemiluminescence detection. Anal Chem 65:1570
Thuman EM, Mills MS (1998) Solid phase extraction, principles and practices. Wiley, New York
Sturgeon RE, Berman SS, Desaulniers A, Russel DS (1980) Pre-concentration of trace metals from sea water for determination by graphite furnace atomic absorption spectrometry. Talanta 27:85
Sarkar AR, Dutta PK, Sarkar M (1996) Sorption recovery of metal ions using silica gel modified with salisilaldoxime. Talanta 43:857
Blain S, Appriov P, Handel H (1991) Column pre-concentration of trace metals from sea water with mesoporous resins impregnated with lipiphilic tetraaza macrocycles. Analyst 116:815
Kumar M, Rathore DPS, Singh AK (2001) Quinalizarin anchored on Amberlite XAD-2, A new matrix for solid phase extraction of metal ions for flame atomic absorption spectrometric determination. Fresenious J Anal Chem 370:377
Mahmoud ME, Soliman EM (1997) Silica immobilised formylsalicylic acid as a selective base for extraction of iron (III). Talanta 4:15
Ramesh A, Rama MK, Seshaiah K (2002) Pre-concentration of trace elements on Amberlite XAD-4 resin coated with dithiocarbamate and determination by inductively coupled plasma—Atomic emission spectrometry in saline water. Talanta 57:243
Zaporozhets O, Gawer O, Sukhan V (1998) Determination of Fe(II), Cu(II), and Ag(I) by using silica gel loaded with 1, 10-phenanthroline. Talanta 46:1387
Isabel DH, Mariano F, Isabel S (2009) Solid phase extraction of Pb(II) in water samples using a new hybrid inorganic-organic meso-porous silica prior to its determination by FAAS. Microchim Acta 166:391
Lijun Z, Qun H, Xinping H, Zheng H (2009) Determination of trace metals in natural samples by ICP-OES after pre-concentration on modified silica gel and on modified silica nano particles. Microchim Acta 169:347
Sereje T, Vedat Y, Senol K, Ali D, Cengiz S (2009) Solid phase extraction of Pd (II) on a newly synthesized chelating resin prior to determination by FAAS. Microchim Acta 165:347
Limin W, Meihua Z, Zhijuan J, Aifu Z (2009) Selective separation of lead from aqueous solution with a novel Pb (II) surface ion-impregnated sol gel sorbent. Microchim Acta 65:367
Lian Z, Xijun C, Zheng H, Lijun Z, Jianping S, Ru G (2010) Selective solid phase extraction and separation of mercury environmental and biological samples by nanometer silica functionalized with 2, 6, pyridine di-carboxylic acid. Microchim Acta 168:79
Curie J (1978) Hand book of anal chemistry. MIR, Moscow
Zaporozhets OA, Tsyukalo LY (2002) Xylenol orange adsorbed on silica surface as a solid phase reagent for lead determination using diffuse reflectance spectroscopy. Talanta 58:861
Jing F, Chunlai W, Yafang W, Chuanyun P, Pingan P (2007) Preparation of xylenol orange functionalized silica gel as a selective solid phase extractor and its application for pre-concentration. J Hazard Mater 145:323
Robbins JC (1978) Field technique for measurement of uranium in natural water. GIM Bulletin 793:61
Whitkop PG (1982) Determination of uranium in aqueous samples by laser induced fluorescence spectrometry. Anal Chem 54:2475
Hong KB, Jung KW, Jung KH (1989) Application of laser induced fluorescence for the determination of trace uranium, europium and samarium. Talanta 36:1095
Eral M, Kinaci SR (1989) Determination of uranium in natural waters by laser exited fluorescence for uranium exploration. Spectrosc Lett 22:855
Campen W, Bachmann K (1979) Laser induced fluorescence for direct determination of small concentration of uranium in water. Mikro Chim Acta 11:159
Creig ZA, Linde CH, Charles PE (1981) Determination of nano gram quantities of uranium by pulsed laser fluorimetry. Mikro Chim Acta 11:457
Nakanishi M (1950) Fluorimetric micro determination of uranium. Bull Chem Soc Jpn 23:198
Balaji BK, Bincy C, Mishra G (1996) A rapid method for the determination of uranium in brines by laser fluorimetric technique. EARFAM 9:99
Smith AP, Grimaldi FS (1954) The fluorimetric determination of uranium in non saline and saline waters Part-17, Collected papers on analysis for uranium and thorium. U.S.Geological survey Bulletin 125–131
Kochan IG, Shuktomova II (1994) Separation of uranium from soil for its determination. J Radioanal Nucl Chem 188:27
Leela G, Nayeem MA, Murty DSR (2007) Biosorption of uranium for separation / pre-concentration prior to its estimation by fluorimetry. EARFAM 17:31
Singh BN, Maiti B (2006) Separation and pre-concentration of U (VI) on XAD-4 modified with 8-hydroxy quinoline. Talanta 69:393
Ansari SA, Mohapatra PK, Manchanda VK (2008) A novel malonamide grafted polystyrene-divinyl benzene resin for extraction, pre-concentration and separation of actinides. J Hazard Mater 04:93
Dojozan D, Pourna Ghiazar MH, Toutounchiasr J (1998) Pre-concentration of trace uranium from sea water with solid phase extraction followed by differential pulse polorographic determination in chloroform eluate. Talanata 46:123
Israclachvilli JN, Pashely RM (1984) Measurement of hydrophobic interaction between two hydrophobic surfaces in aqueous electrolyte solution. J Colloid Interface Sci 98:500
Acknowledgements
The authors are thankful to the Shri.A.K.Rai, Regional Director, Southern Region, AMD, Bangalore; Additional Director (OP-I) and Additional Director (R&D), AMD, Hyderabad, for their encouragement and providing facilities to carry out this work. The authors are thankful to Dr. K.Satyanarayana, SO/G, Head, Chemistry Group, AMD, Hyderabad and Dr.A. Premadas SO/G, Chemistry Laboratory, Bangalore for their constant encouragement and technical advice. Authors are also grateful to the Director, AMD for presentation/publication of the investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cyriac, B., Balaji, B.K. A novel method of synthesizing solid phase adsorbent silica modified with xylenol orange: application for separation, pre-concentration and determination of uranium in calcium rich hydro-geochemical samples and sea water—Part 1. Microchim Acta 171, 33–40 (2010). https://doi.org/10.1007/s00604-010-0369-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0369-2