Abstract
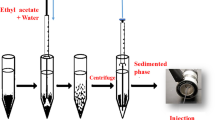
A fast, sensitive and simple oil-in-water emulsion (OWE) method was developed for extraction of four phenolic pollutants in environmental water samples followed by gas chromatography and flame ionization detection. In this method, the density of a binary organic solvent (one heavier and one lighter than the sample) was balanced with the density of the sample solution. A stable emulsion was formed at room temperature under vigorous stirring using a Teflon-coated magnetic stirring bar. After addition of 10 µL of the heavier organic solvent and centrifugation, phase separation occurred. The influence of several important parameters on the extraction efficiency of phenolic compounds was evaluated. Under optimized experimental conditions, the calibration graphs were linear in the concentration range 0.025–20 mg L−1 with coefficients of determination more than 0.9994. The limits of detection and quantification were in the range 19.2–76.0 and 64.1–251.0 μg L−1, respectively. Intra-day and inter-day precisions were less than 5.0 %. The procedure was used for the determination of phenolic compounds in spiked water samples with good results. Recoveries range from 96.5 to 103.0%, and relative standard deviations are <2.5% (for n = 3).




Similar content being viewed by others
References
Liu X, Ji Y, Zhang Y, Liu H (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165:10
Shen S, Chang Z, Liu H (2006) Three-liquid-phase extraction system for separation of phenol and p-nitrophenol from wastewater. Sep Pur Technol 49:17
Saraji M, Bakhshi M (2005) Determination of phenols in water samples by single-drop microextraction followed by in-syringe derivatization and gas chromatography-mass spectrometric detection. J Chromatogr A 1098:30
Andres MPS, Leon-Gonzalez ME, Perez-Arribas LV, Polo-Diez LM (2000) Determination of pollutant phenols by capillary high-performance liquid chromatography with UV-detection. J High Resol Chromatogr 23:367
Almeda S, Nozal L, Arce L, Valcarcel M (2007) Direct determination of chlorophenols present in liquid samples by using a supported liquid membrane coupled in-line with capillary electrophoresis equipment. Anal Chim Acta 587:97
Reis MTA, Freitas OMF, Ismael MRC, Carvalho JMR (2007) Recovery of phenol from aqueous solutions using liquid membranes with Cyanex 923. J Membr Sci 305:313
Sirvent G, Sanchez JM, Salvado V (2004) Preconcentration and determination of priority pollutant phenols in waters at trace levels using a polymeric solid-phase extraction cartridge. J Sep Sci 27:1524
Zhou F, Li X, Zeng Z (2005) Determination of phenolic compounds in wastewater samples using a novel fiber by solid-phase microextraction coupled to gas chromatography. Anal Chim Acta 538:63
Penalver A, Pocurull E, Borrull F, Marce RM (2002) Solid-phase microextraction coupled to high-performance liquid chromatography to determine phenolic compounds in water samples. J Chromatogr A 953:79
Fouad EA, Bart HJ (2008) Emulsion liquid membrane extraction of zinc by a hallow-fiber contactor. J Membr Sci 307:156
Othman N, Mat H, Goto M (2006) Separation of silver from photographic wastes by emulsion liquid membrane system. J Membr Sci 282:171
Kumbasar RA (2009) Selective extraction and concentration of cobalt from acidic leach solution containing cobalt and nickel through emulsion liquid membrane using PC-88A as extractant. Sep Pur Technol 64:273
Mirhosseini H, Tan CP, Hamid NSA, Yusof S, Chern BH (2009) Characterization of the influence of main emulsion components on the physicochemical properties of orange beverage emulsion using response surface methodology. Food Hydrocolloids 23:271
Domenico GA, Massimo MA, Sanzo RDA, Rossi COB, Silverstro A, Ruffolo B, Bruno de Cindio A (2009) Characterization of dairy emulsions by NMR and rheological techniques. Food Hydrocolloids 23:619
Meloan CE (1999) Chemical separations: principles, techniques, and experiments, John Wiley & Sons, INC, p. 451
Ashby NP, Binks BP (2000) Pickering emulsion stabilised by laponite clay particles. Phys Chem Chem Phys 2:5640
Tadros T, Izquierdo P, Esquena J, Solans C (2004) Formation and stability of nano-emulsion. Adv Colloid Interface Sci 108–109:303
Hoang TKN, La VB, Deriemaeker L, Finsy R (2004) Ostwald ripening and solubilization in alkane in water emulsions stabilized by different surfactants. Phys Chem Chem Phys 6:1413
Chen G, Tao D (2005) An experimental study of stability of oil-water emulsion. Fuel Process Technol 86:499
Fiamegos YC, Nanos CG, Pilidis GA, Stalikas CD (2003) Phase-transfer catalytic determination of phenols as methylated derivatives by gas chromatography with flame ionization and mass-selective detection. J Chromatogr A 983:215
Ruiz-Jimenez J, Luque de Castro MD (2007) In-column micro-high-performance liquid chromatographic concentration-separation prior to ultraviolet detection for the determination of chlorophenols in water samples. J Chromatogr A 1174:78
Bagheri H, Saber A, Mousavi SR (2004) Immersed solvent microextraction of phenol and chlorophenols from water samples followed by gas chromatography-mass spectrometry. J Chromatogr A 1046:27
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Characteristic parameters of the calibration equations for the proposed oil-in-water emulsion method for simultaneous analysis of phenolic compounds. (DOC 23 kb)
Table S2
Results from determination of within-day and between-day precision of phenolic compounds. (DOC 41 kb)
Fig. S1
Effect of stirring speed on the extraction efficiency of phenolic compounds. Extraction conditions: sample volume = 5 mL aqueous solution containing 2% NaCl and 0.01 mol L−1 HCl (pH 2); heavier phase volume = 20.2 µL CCl4; lighter phase volume = 79.8 µL of butyl acetate; extraction time = 1 min; temperature = 23°C. (DOC 184 kb)
Fig. S2
Effect of temperature on the extraction efficiency of phenolic compounds. Extraction conditions: sample volume = 5 mL aqueous solution containing 2% NaCl and 0.01 mol L−1 HCl (pH 2); heavier phase volume = 20.2 µL CCl4; lighter phase volume = 79.8 µL of butyl acetate; stirring rate = 700 rpm; extraction time = 1 min. (DOC 181 kb)
Rights and permissions
About this article
Cite this article
Daneshfar, A., Khezeli, T. Extraction of phenolic compounds from environmental water samples using oil-in-water emulsions. Microchim Acta 167, 211–216 (2009). https://doi.org/10.1007/s00604-009-0242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0242-3