Abstract
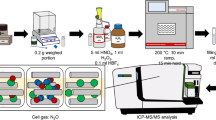
The effects of calcium and magnesium (as nitrates) and phosphorous (as hydrogen phosphate) were investigated on the stability of As, Cd, Hg, and Se during open-vessel dissolution in Teflon vessels. Samples of mainly inorganic and biological matrices were dissolved in screw-capped Teflon tubes in HNO3 only or in a mixture of HNO3-HF. The caps were then removed and the solutions were simultaneously evaporated at 120 °C to near dryness without drying the contents (Method I) or to complete dryness with extended heating for 20 min at dryness (Method II). ICP-MS analysis indicated that the stabilities of Se and Hg were highly influenced by Ca, Mg and PO4 content in the sample. Arsenic (As) and Cd did not show any significant instability or volatility. Selenium was lost in Method II from biological samples containing trace levels of Ca, Mg and PO4. Mercury was unstable during heating in all samples, except bone ash for which no significant loss was detected in Method I. Losses observed for Hg and Se were consistent with Ca, Mg and PO4 deficiency in the samples and hence indicated that nitrate and hydrogen phosphate salts of these matrix elements do improve stability of the relatively volatile elements during open-vessel dissolution in teflon vessels. While Se was effectively stabilized with sub-per cent levels of Ca, Mg and PO4, Hg due its high volatility required significantly higher levels of Ca and PO4 in the bone ash.


Similar content being viewed by others
References
Clement RE, Yang PW, Koester CJ (2001) Environmental analysis. Anal Chem 73:2761
Bings NH, Bogaerts A, Broekaert JAC (2004) Atomic spectroscopy. Anal Chem 76:3313
Richardson SD (2004) Environmental mass spectrometry: emerging contaminants and current issues. Anal Chem 76:3337
Beauchemin D (2004) Inductively coupled plasma mass spectrometry. Anal Chem 76:3395
Sun YC, Chi PH, Shiue MY (2001) Comparison of different digestion methods for total decomposition of siliceous and organic environmental samples. Anal Sci 17:1395
Randa PJ, Spencer K (1980) Sulfur content of plant material: comparison of oxidation prior to determination. Commun Soil Sci Plant Anal 11:257
Somer G, Ünlü AN (2006) The effect of acid digestion on the recoveries of trace elements: Recommended policies for the elimination of losses. Turk J Chem 30:745
Vassileva E, Docekalova H, Baeten H, Vanhentenrijk S, Hoenig M (2001) Revisitation of mineralization modes for arsenic and selenium determinations in environmental samples. Talanta 54:187
Rodushkin L, Tuth T, Hubtassari A (1999) Comparison of two digestion methods for elemental determinations in plant material by ICP techniques. Anal Chim Acta 378:191
Hoenig M, Kersabiec AM (1996) Sample preparation steps for analysis by atomic spectroscopy methods: present status. Spectrochim Acta 51B:1297
Arslan Z, Ertas N, Tyson JF, Uden PC, Denoyer E (2000) Determination of trace elements in marine plankton by inductively coupled plasma mass spectrometry (ICP-MS). Fresenius J Anal Chem 366:273
Lamble KJ, Hill SJ (1998) Microwave digestion procedures for environmental matrices: critical review. Analyst 123:103R
Kingston HM, Jassie LB (eds) (1988) Introduction to microwave sample preparation: theory and practice. ACS, Washington
Campbell MJ, Törvenyi A (1999) Non-spectroscopic suppression of zinc in ICP-MS in a candidate biological reference material (IAEA 392 Algae). J Anal At Spectrom 14:1313
Volynskii AB (2003) Chemical modifiers in modern electrothermal atomic absorption spectrometry. J Anal Chem 58:1061
Nobrega JA, Rust J, Calloway CP, Jones BT (2004) Use of modifiers with metal atomizers in electrothermal atomic absorption spectrometry: a short review. Spectrochim Acta 59B:1337
Arslan Z, Tyson JF (2007) Slurry sampling for determination of lead in marine plankton by electrothermal atomic absorption spectrometry. Microchem J 86:227
Bermejo-Barrera P, Moreda-Pineiro J, Moreda-Pineiro A, Bermejo-Barrera A (1996) Comparative study of magnesium nitrate, palladium nitrate and reduced palladium for the direct determination of mercury in sea water by electrothermal atomization atomic absorption spectrometry. Mikrochim Acta 124:111
Bermejo-Barrera P, Barciela-Alonso MC, Moreda-Pineiro J, Gonzalez-Sixto C, Bermejo-Barrera A (1996) Determination of trace metals (As, Cd, Hg, Pb and Sn) in marine sediment slurry samples by electrothermal atomic absorption spectrometry using palladium as a chemical modifier. Spectrochim Acta 51B:1235
Imai S, Sugimoto A, Hayashi M, Yonetani A, Ewamoto E, Hayashi Y (2000) Comparison of chemical modifiers in electrothermal atomic absorption spectrometry with boron nitride tube and platform furnaces for mercury. Anal Sci 16:739
Da Silva AF, Welz B, Curtius AJ (2002) Noble metals as permanent chemical modifiers for the determination of mercury in environmental reference materials using solid sampling graphite furnace atomic absorption spectrometry and calibration against aqueous standards. Spectrochim Acta 57B:2031
Saber-Tehrani M, Hashemi-Moghaddam H, Givianrad MH, Abroomand-Azar P (2006) Methylmercury determination in biological samples using electrothermal atomic absorption spectrometry after acid leaching extraction. Anal Bioanal Chem 386:1407
Lopez-Garcia I, Sanchez-Merlos M, Hernandez-Cordoba M (1997) Determination of mercury in soil and sediments by graphite furnace atomic absorption spectrometry with slurry sampling. Spectrochim Acta 52B:2085
Bermejo-Barrera P, Moreda-Pineiro J, Moreda-Pineiro A, Bermejo-Barrera A (1996) Comparison of different chemical modifiers for the direct determination of arsenic in sea water by electrothermal atomic absorption spectrometry. Fresenius J Anal Chem 355:174
Ferraresi V, Fornasari P (1997) Determination of boron by graphite furnace AAS: Comparison of different modifiers. AA Instruments at work (www.varian.com) AA-125:1
Quevauviller P, Vercoutere K, Muntau H, Griepink B (1993) Certified reference material (CRM 414) for the quality control of trace element analysis in plankton. Fresenius J Anal Chem 345:12
Acknowledgements
This work is funded in part by grants from NIH-RCMI Program (Grant No G12RR013459) and NIH-ERDA Program (Grant No 5 G11 HD046519-05) to Jackson State University. The views expressed herein are those of authors and do not necessarily represent the official views of the NIH and any of its sub-agencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afonso, D.D., Arslan, Z. & Bednar, A.J. Assessment of matrix-dependent analyte stability and volatility during open-vessel sample dissolution for arsenic, cadmium, mercury and selenium. Microchim Acta 167, 53 (2009). https://doi.org/10.1007/s00604-009-0218-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-009-0218-3