Abstract
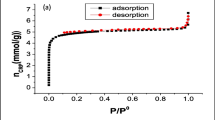
A novel Pb(II) ion-imprinted mesoporous sorbent (IIMS) was synthesized by a surface imprinting technique combined with a sol-gel process and characterized by FT-IR and N2 adsorption-desorption. Compared to the non-imprinted mesoporous sorbent (NIMS), the IIMS had a higher adsorption capacity and selectivity for Pb(II). The maximum static adsorption capacities of the IIMS and NIMS for Pb(II) were 221 and 173 mg g−1, respectively. The relative selectivity coefficients of the sorbent for Pb(II) in the presence of Cd(II), Cu(II) and Zn(II) were 3.7, 1.9 and 3.4, respectively. Furthermore, the IIMS possessed a fast kinetics for Pb(II) sorption from aqueous solution with saturation time of < 20 min, and could be used repeatedly. The detection limit (3σ) of this method was 0.23 ng mL−1 and relative standard deviation of 11 replicate determinations was 3.7 %. The IIMS has been applied to selectively separate and determine Pb(II) in real water samples with satisfactory results.




Similar content being viewed by others
References
Benaroya RO, Tzin V, Tel-Or E et al (2004) Lead accumulation in the aquatic fern Azolla filiculoides. Plant Physiol Bioch 42:639–645
Macías-García A, Valenzuela-Calahorro C, Espinosa-Mansilla A et al (2004) Adsorption of Pb2+ in aqueous solution by SO2-treated activated carbon. Carbon 42:1755–1764
Zhan XM, Zhao X (2003) Mechanism of lead adsorption from aqueous solutions using an adsorbent synthesized from natural condensed tannin. Water Res 37:3905–3912
Dolgopolova A, Weiss DJ, Seltmann R et al (2004) Closed-vessel microwave digestion technique for lichens and leaves prior to determination of trace elements (Pb, Zn, Cu) and stable Pb isotope ratios. Int J Environ Anal Chem 84:889–899
Petit de Peña Y, Paredes B, Rondón W et al (2004) Continuous flow system for lead determination by faas in spirituous beverages with solid phase extraction and on-line copper removal. Talanta 64:1351–1357
Boonamnuayvitaya V, Chaiya CY, Tanthapanichakoon W et al (2004) Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep Pur Technol 35:11–22
Shan HX, Li ZJ, Li M (2007) Ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate as a solvent for extraction of lead in environmental water samples with detection by graphite furnace atomic absorption spectrometry. Microchim Acta 159:95–100
Lu YK, Yan XP (2004) An imprinted organic-inorganic hybrid sorbent for selective separation of cadmium from aqueous solution. Anal Chem 76:453–457
Rao TP, Kala R, Daniel S (2001) Metal ion-imprinted polymers—Novel materials for selective recognition of inorganics. Anal Chim Acta 578:105–116
Zheng H, Zhang D, Wang WY et al (2007) Highly selective determination of palladium(II) after preconcentration using Pd(II)-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Microchim Acta 157:7–11
Chang XJ, Wang XY, Jiang N et al (2008) Silica gel surface-imprinted solid-phase extraction of Zr(IV) from aqueous solutions. Microchim Acta 162:113–119
Wang S, Zhang RF (2006) Selective Solid-Phase Extraction of Trace Copper Ions in Aqueous Solution with a Cu(II)-Imprinted Interpenetrating Polymer Network Gel Prepared by Ionic Imprinted Polymer (IIP) Technique. Microchim Acta 154:73–80
Metilda P, Prasad K, Kala R et al (2007) Ion imprinted polymer based sensor for monitoring toxic uranium in environmental samples. Anal Chim Acta 582:147–153
Ye L, Mosbach K (2001) Polymers recognizing biomolecules based on a combination of molecular imprinting and proximity scintillation: A new sensor concept. J Am Chem Soc 123:2901–2902
Pan J Y, Wang S, Zhang R F(2006) A novel Pb(II)-imprinted IPN for selective preconcentration of lead from water and sediments. Int J Environ Anal Chem 86: 855–865.
Rao TP, Daniel S, Gladis J M (2004) Tailored materials for preconcentration or separation of metals by ion-imprinted polymers for solid-phase extraction (IIP-SPE). Trends Anal Chem 23:28–36
Na J, Chang XJ, Hong Z et al (2006) Selective solid-phase extraction of nickel(II) using a surface-imprinted silica gel sorbent. Anal Chim Acta 577:225–231
Han DM, Fang GZ, Yan XP (2005) Preparation and evaluation of a molecularly imprinted sol-gel material for on-line solid-phase extraction coupled with high performance liquid chromatography for the determination of trace pentachlorophenol in water samples. J Chromatogr A 1100:131–136
Dai S, Burleigh MC, Shin Y et al (1999) Imprint coating: a novel synthesis of selective functionalized ordered mesoporous sorbents. Angew Chem Int Ed 38:1235–1239
Fang GZ, Tan J, Yan XP (2005) An Ion-Imprinted Functionalized Silica Gel Sorbent Prepared by a Surface Imprinting Technique Combined with a Sol-Gel Process for Selective Solid-Phase Extraction of Cadmium(II). Anal Chem 77:1734–1739
Fan ZF (2006) Hg(II)-imprinted thiol-functionalized mesoporous sorbent micro-column preconcentration of trace mercury and determination by inductively coupled plasma optical emission spectrometry. Talanta 70:1164–1169
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Barret EP, Joyner LG, Halenda PP (1951) The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J Am Chem Soc 73:373–380
Mapolelo M, Torto N (2004) Trace enrichment of metal ions in aquatic environments by Saccharomyces cerevisiae. Talanta 64:39–47
Sekhar KC, Kamala CT, Chary NS et al (2003) Removal of heavy metals using a plant biomass with reference to environmental control. Int J Miner Process 68:37–45
Long GL, Winefordner JD (1983) Limit of detection, a closer look at the IUPAC definition. J Anal Chem 55:712A–724A
Dadfarnia S, Salmanzadeh AM, Shabani AMH et al (2008) A novel separation/preconcentration system based on solidification of floating organic drop microextraction for determination of lead by graphite furnace atomic absorption spectrometry. Anal Chim Acta 623:163–167
Acknowledgement
This work was supported by Shanghai Leading Academic Discipline Project, Project Number : B604.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Zhou, M., Jing, Z. et al. Selective separation of lead from aqueous solution with a novel Pb(II) surface ion-imprinted sol-gel sorbent. Microchim Acta 165, 367–372 (2009). https://doi.org/10.1007/s00604-009-0146-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0146-2