Abstract
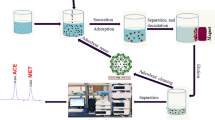
A novel column solid phase extraction method was developed for the determination of Cu(II) and Fe(III) in various samples by flame atomic absorption spectrometry (FAAS) after preconcentration as their N-benzoyl-N-phenylhydroxylamine complexes on Amberlite XAD-1180 resin. Elution was performed with 1 mol L−1 HCl in acetone. The effects of some analytical parameters such as pH, amount of reagent, eluent type, concentration and volume, flow rate of sample and elution solutions, volume of the sample solution and matrix interference were investigated. The recoveries of Cu(II) and Fe(III) under optimum conditions were ≥95%. A good relative standard deviation (≤2.0%), a good preconcentration factor (50) and low detection limits (0.82 μg L−1 for Cu and 1.05 μg L−1 for Fe) were obtained. The accuracy of the procedure developed was confirmed by determining the analytes in three certified reference materials (TMDA-54.4 fortified lake water, SRM 1643e trace elements in water, SRM 1568a rice flour) and the analysis of spiked water samples. The results showed a good agreement with the certified values. The method was applied to the determination of the analytes in water (sea water, dam water, lake water and waste water), vegetable, rice and spices samples.


Similar content being viewed by others
References
Mondal BC, Das D, Das AK (2002) Preconcentration and separation of copper, zinc and cadmium by the use of 6-mercapto purinylazo resin and their application in microwave digested certified biological samples followed by AAS determination of the metal ions. J Trace Elem Med Biol 16:145
Yamini Y, Tamaddon A (1999) Solid-phase extraction and spectrophotometric determination of trace amounts of copper in water samples. Talanta 49:119
Tokman N (2007) The use of slurry sampling for the determination of manganese and copper in various samples by electrothermal atomic absorption spectrometry. J Hazard Mater 143:87
Lemos VA, Santos ES, Gama EM (2007) A comparative study of two sorbents for copper in a flow injection preconcentration system. Sep Purif Technol 56:212
Xiong C, Jiang Z, Hu B (2006) Speciation of dissolved Fe(II) and Fe(III) in environmental water samples by micro-column packed with N-benzoyl-N-phenylhydroxylamine loaded on microcrystalline naphthalene and determination by electrothermal vaporization inductively coupled plasma-optical emission spectrometry. Anal Chim Acta 559:113
Liang P, Chen X (2005) Preconcentration of rare earth elements on silica gel loaded with 1-phenyl-3-methyl-4-benzoylpyrazol-5-one prior to their determination by ICP-AES. Anal Sci 21:1185
Shamspur T, Sheikhshoaie I, Mashhadizadeh MH (2005) Flame atomic absorption spectroscopy (FAAS) determination of iron (III) after preconcentration on to modified analcime zeolite with 5-((4-nitrophenylazo)-N-(2′,4′-dimethoxyphenyl)salicylaldimien by column method. J Anal At Spectrom 20:476
Tokalıoğlu Ş, Oymak T, Kartal Ş (2004) Determination of palladium in various samples by atomic absorption spectrometry after preconcentration with dimethylglyoxime on silica gel. Anal Chim Acta 511:255
Cid BP, Segade SR, Bendicho C (1997) Determination of copper in mineral waters from Galicia, Spain, by flame atomic absorption spectrometry using preconcentration with diethyldithiocarbamate loaded on silica gel. Microchem J 55:319
Jiang Y, Wu Y, Liu J, Xia X, Wang D (2008) Ammonium pyrrolidinedithiocarbamate-modified activated carbon micro-column extraction for the determination of As(III) in water by graphite furnace atomic absorption spectrometry. Microchim Acta 161:137
dos Santos EJ, Herrmann AB, Ribeiro AS, Curtius AJ (2005) Determination of Cd in biological samples by flame AAS following on-line preconcentration by complexation with O,O-diethyldithiophosphate and solid phase extraction with Amberlite XAD-4. Talanta 65:593
Baytak S, Balaban A, Türker AR, Erk B (2006) Atomic absorption spectrometric determination of Fe(III) and Cr(III) in various samples after preconcentration by solid phase extraction with pyridine-2-carbaldehyde thiosemicarbazone. J Anal Chem 61:476
El-Shahat MF, Moawed EA, Farag AB (2007) Chemical enrichment and separation of uranyl ions in aqueous media using novel polyurethane foam chemically grafter with different basic dyestuff sorbents. Talanta 71:236
Pyrzyñska K, Trojanowicz M (1999) Functionalized cellulose sorbents for preconcentration of trace metals in environmental analysis. Crit Rev Anal Chem 29:313
Pancras JP, Puri BK (2002) Column preconcentration and FAAS determination of copper, iron, nickel and zinc using 2-(5-bromo-2-pyridylazo)-5-diethylamino-phenoltetraphenylborate-naphthalene adsorbent. Anal Bioanal Chem 374:1306
Żylkiewicz BG (2003) Biosorption of platinum and palladium for their separation/preconcentration prior to graphite furnace atomic absorption spectrometric determination. Spectrochim Acta 58:1531
Zhai Y, Chang X, Cui Y, Lian N, Lai S, Zhen H, He Q (2006) Selective determination of trace mercury (II) after preconcentration with 4-(2-pyridylazo)-resorcinol-modified nanometer-sized SiO2 particles from sample solutions. Microchim Acta 154:253
Zhu X, Yang D, Chang X, Cui Y, Hu Z, Zou X (2008) Selective solid-phase extraction of trace Fe(III) from biological and natural water samples using nanometer SiO2 modified with acetylsalicylic acid. Microchim Acta (in press)
Pourreza N, Hoveizavi R (2005) Simultaneous preconcentration of Cu, Fe and Pb as methylthymol blue complexes on naphthalene adsorbent and flame atomic absorption determination. Anal Chim Acta 549:124
Garbos S, Bulska E, Hulanicki A, Fijałek Z, Sołtyk K (2000) Determination of total antimony and antimony (V) by inductively coupled plasma mass spectrometry after selective separation of antimony (III) by solvent extraction with N-benzoyl-N-phenylhydroxylamine. Spectrochim Acta Part B 55:795
Robles LC, Olalla CG, Alemany MT, Aller AJ (1991) Flame atomic absorption spectrometric method for the determination of beryllium in natural waters after separation with N-benzoyl-N-phenylhydroxylamine. Analyst 116:735
Ueno K, Imamura T, Cheng KL (1992) Handbook of organic analytical reagents. CRC, Boca Raton, FL, p 127
Yang XJ, Pin C (2002) Determination of niobium, tantalum, zirconium and hafnium in geological materials by extraction chromatography and inductively coupled plasma mass spectrometry. Anal Chim Acta 458:375
Tokalıoğlu Ş, Oymak T, Kartal Ş (2007) Coprecipitation of lead and cadmium using copper(II) mercaptobenzothiazole prior to flame atomic absorption spectrometric determination. Microchim Acta 159:133
Ramesh A, Devi BA, Hasewaga H, Maki T, Ueda K (2007) Nanometer-sized alumina coated with chromotropic acid as solid phase metal extractant from environmental samples and determination by inductively coupled plasma atomic emission spectrometry. Microchem J 86:124
Hseu Z-Y (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour Technol 95:53
Acknowledgments
The authors are grateful for the financial support of the Unit of the Scientific Research Projects of Erciyes University (Project No: EUBAP-FBT-06-05).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM
(DOC 57.5 KB)
Rights and permissions
About this article
Cite this article
Tokalıoğlu, Ş., Livkebabcı, A. A new solid-phase extraction method for the determination of Cu(II) and Fe(III) in various samples by flame atomic absorption spectrometry using N-benzoyl-N-phenylhydroxylamine. Microchim Acta 164, 471–477 (2009). https://doi.org/10.1007/s00604-008-0090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0090-6