Abstract
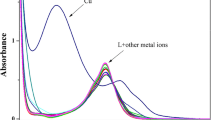
A new luminol derivative has been synthesized and its fluorescence has been studied. The luminol derivative emits at 460 nm in pH 10.0 phosphate buffer which is different from the original luminol that emits at 425 nm under the same conditions. Cu2+ exhibits strong fluorescence quenching effect on the luminol derivative and the quenching mechanism is discussed in detail. This luminol derivative can be applied as a fluorescent probe for ultra-trace analysis of Cu2+. The effects of pH, interferents and the stability have also been investigated. The fluorescent probe shows good reproducibility, and the relative standard deviation of response to Cu2+ is 0.7% (n = 6). The lower linear range and the limit of detection are 0–500 and 0.27 nmol L−1, respectively.




Similar content being viewed by others
References
Badocco D, Pastore P, Favaro G, Maccà C (2007) Effect of eluent composition and pH and chemiluminescent reagent pH on ion chromatographic selectivity and luminol-based chemiluminescence detection of Co2+, Mn2+ and Fe2+ at trace levels. Talanta 72:249
Li B, Wong D, Lv J, Zhang Z (2006) Flow-injection chemiluminescence simultaneous determination of cobalt(II) and copper(II) using partial least squares calibration. Talanta 69:160
Burguera JL, Burguera M, Townshend A (1981) Determination of zinc and cadmium by flow injection analysis and chemiluminescence. Anal Chim Acta 127:199
Hou XF, Zhang ZJ, Zhao Y, Ma J (2007) Microdialysis sampling and chemiluminescence detection for in vivo and real-time study of the lead metabolism in rabbit blood. Microchimica Acta 159:223
Marina-Sánchez MA, Díaz-García ME, Sanz-Medel A (1992) Simultaneous determination of cobalt and chromium by ion chromatography with chemiluminescence detection and its application to glass analysis. Microchimica Acta 106:227
Burdo G, Seitz WR (1975) Mechanism of cobalt catalysis of luminol chemiluminescence. Analytical Chemistry 47:1693
Burguera JL, Townshend A (1981) Determination of manganese(II) by a chemiluminescence reaction. Talanta 28:731
Haapakka KE, Kankare JJ (1980) Application of the electrochemiluminescence of luminol to the determination of copper. Anal Chim Acta 118:333
Seitz W, Hercules D (1972) Determination of trace amounts of iron(II) using chemiluminescence analysis. Analytical Chemistry 44:2143
Yoshida H, Ureshino K, Ishida J, Nohta H, Yamahnchi M (1999) Chemiluminescent properties of some luminol related compounds (II). Dyes Pigments 41:177
Hirata S, Hashimoto Y, Aihara M, Mallika GV (1996) On-line column preconcentration for the determination of cobalt in sea water by flow-injection chemiluminescence detection. Fresenius J Anal Chem 355:676
Lannuzel D, Jong J, Schoemann V, Trevena A, Tison JL, Chou L (2006) Development of a sampling and flow injection analysis technique for iron determination in the sea ice environment. Anal Chim Acta 556:476
Bowie AR, Achterberg EP, Sedwick PN, Ussher S, Worsfold PJ (2002) Real-time monitoring of picomolar concentrations of iron(II) in marine waters using automated flow injection-chemiluminescence instrumentation. Environ Sci Technol 36:4600
Cannizzaro V, Bowie AR, Sax A, Acheterberg EP, Worsfold PJ (2000) Determination of cobalt and iron in estuarine and coastal waters using flow injection with chemiluminescence detection. Analyst 125:51
Watanabe F, Miyamoto E (2002) TLC separation and analysis of vitamin B-12 and related compounds in food. J Liq Chromatogr Relat Technol 25:1561
Banerjee R, Ragsdale SW (2003) The many faces of vitamin B-12: catalysis by cobalamin-dependent enzymes. Ann Rev Biochem 72:209
Huang XY, Ren JC (2006) Chemiluminescence detection for capillary electrophoresis and microchip capillary electrophoresis. Trends Anal Chem 25:155
Will G, Kudryashov E, Duggan E, Fitzmaurice D, Buckin V, Waghorne E, Mukherjee S (1999) Excited state complex formation between 3-aminophthalhydrazide and DNA: a fluorescence quenching reaction. Spectrochim Acta, Part A: Mol Biomol Spectrosc 55:2711
Banerjee D, Mandal A, Mukherjee S (2003) Excited state complex formation between methyl glyoxal and some aromatic bio-molecules: a fluorescence quenching study. Spectrochim Acta, Part A: Mol Biomol Spectrosc 59:103
Guha D, Bhattacharjee U, Mitra S, Das R, Mukherje S (1998) Interaction of 3-aminophthalhydrazide with 5-hydroxytetracycline and chloramphenicol: a fluorescence quenching study. Spectrochim Acta, Part A: Mol Biomol Spectrosc 54:525
Ma DL, Cui FL, Xia DS, Wang YL (2002) Spectrophotometric determination of copper and palladium using a new reagent. Analytical Letter 35:413
Ma DL, Ding GS, Wang CX, Zhang L, Wang YL (2000) The characterization of a new reagent, N-allyl-N′-(sodium p-aminobenzenesulfonate) thiourea, and its application to determination of Pd(II). Analytical Letters 33:2533
Ma DL, Xia DS, Cui FL, Li JP, Wang YL (1999) A new sensitive reagent for identifying and determining Cu2+. Talanta 48:9
Ma DL, Li Y, Li QJ, Wang YL (2001) Spectrophotometric determination of palladium(II) with new reagent N-octyl-N′-(sodium p-amminobenzenesulfonate)thiourea (OPT). J Chin Chem Soc 48:1111
Ma DL, Huang YR, Xia DS, Wang YL (1999) The characterization of a new reagent and its application in determining Cu2+. J Chi Chem Soc 46:933
Ma DL, Li Y, Ma KB, Li JP, Chen JG, Yan JW, Wang YL (2001) A high-selectivity spectrophotometric reagent for determining platinum(IV). Talanta 53:937
White EH, Roswell DF (1970) Chemiluminescence of organic hydrazides. Acc Chem Res 3:54
White EH, Matsuo K (1967) Synthesis and chemiluminescence of an amino derivative and sulfur analog of luminol. J Org Chem 32:1921
Merényi G, Lind J, Eriksen TE (1990) Luminol chemiluminescence: chemistry, excitation, emitter. J Biolumin Chemilumin 5:53
Parker CA, Rees WT (1960) Correction of fluorescence spectra and measurement of fluorescence quantum efficiency. Analyst 85:587
Donna CJ, Stanbury DM (1996) Equilibrium and redox kinetics of copper(II)-thiourea complexes. Inorg Chem 35:3210
Garipov RR, Shtyrlin VG, Safin DA, Zyavkina YI, Sokolov FD, Konkin AL, Aganov AV, Zakharov AV (2006) Combined EPR and DFT study of the copper(II) complexes with N-phosphoryl thioureas. Chem Phys 320:59
Westa DX, Szczepurab LF, Giesena JM, Kaminskya W, Kelleyb J, Goldberga KI (2003) Oxidation of heterocyclic thioureas to form benzothiazoles and their copper(II) complexes. J Mol Struct 646:95
Zhang Y, Zhang H, Guo X, Wang H (2008) L-Cysteine-coated CdSe/CdS core-shell quantum dots as selective fluorescence probe for copper(II) determination. Microchem J 89:142
Cano-Raya C, Fernández-Ramos MD, Capitán-Vallvey LF, Wolfbeis OS, Schaferling M (2005) Fluorescence quenching of the europium tetracycline hydrogen peroxide complex by copper(II) and other metal ions. Appl Spectrosc 59:1209
Mei L, Xiang Y, Tong A (2007) A new fluorescent probe of rhodamine B derivative for the detection of copper ion. Talanta 72:1717
Chen X, Ma H (2006) A selective fluorescence-on reaction of spiro form fluorescein hydrazide with Cu(II). Anal Chim Acta 575:217
Cano-Raya C, Fernández-Ramos MD, Capitán-Vallvey LF (2005) Fluorescence resonance energy transfer disposable sensor for copper(II). Anal Chim Acta 555:299
Acknowledgement
This study was supported by grants from National Natural Science Foundation of China (20570542) and the Innovation Foundation of Sichuan University (2006G006).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to electronic supplementary material
ESM 1
(DOC 115 kb)
Rights and permissions
About this article
Cite this article
Luo, Y., Li, Y., Lv, B. et al. A new luminol derivative as a fluorescent probe for trace analysis of copper(II). Microchim Acta 164, 411–417 (2009). https://doi.org/10.1007/s00604-008-0076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0076-4