Abstract
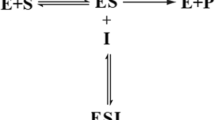
A mathematical model is developed for the simulation of the amperometric response of a biosensor for catechol using polyphenoloxidase. The model is based on transient diffusion equations containing nonlinear terms of Michaelis-Menten for two space regions: the diffusion layer and the biomembrane containing the immobilized enzyme. The set of partial derivatives of nonlinear equations and the corresponding boundary and initial conditions was solved using the implicit finite difference technique. This numerical solution was then exploited to study the effects of permeability and thickness of the biomembrane on the maximum response of the reduction current and the amplification factor corresponding to the maximum of catalytic activity of the enzyme. This amplification factor increases with the thickness of the biomembrane while permeability is weak. In the case of the low initial concentrations (10−6 to 5.10−4 mM), its value is maximal and remains independent of substrate concentration. Also, the amplification factor is more significant when the diffusion resistance is more important, i.e. for high thicknesses or weak permeabilities of the biomembranes.







Similar content being viewed by others
References
Blum LG, Coulet PR (1991) Biosensors principles and applications. Marcel Dekker, New York
Thévenot DR, Toth K, Durst RA, Wilson GS (1999) Electrochemical biosensors: recommended definitions and classification. Pure Appl Chem 2333:71, n°12
Stanca SE, Popescu IC, Oniciu L (2003) Biosensors for phenol derivatives using biochemical signal amplification. Talanta 61:501
Wang Y, Zhu J, Zhu R, Zhu Z, Lai Z, Chen Z (2003) Chitosan/prussian blue-based biosensors. Meas Sci Technol 831:14
Tembe S, Karave M, Inamdas S, Haram S, Melo J, D’souza SF (2006) Development of electrochemical biosensor based on tyrosinase immobilised in composite biopolymeric film. Anal Biochim 349:72
Sanchez-Paniaga Lopez M, Lopez-Cabarcos E, Lopez-Ruiz B (2006) Organic phase electrodes. Biomolecular Engineering 135:23
Shan D, Mousty C, Cosnier S (2003) Layered double hydroxides: an attractive material for electrochemical biosensor design. Anal Chem 3872:75
Tembe S, Inamdas S, Haram S, Karave M, D’souza SF (2007) Electrochemical biosensor for catechol using agar-guargum entrapped tyrosinase. J Biotechnol 128:80
Shan D, Zhu M, Han E, Xue H, Cosnier S (2007) Calcium carbonate nanoparticles: a host matrix for the construction of highly sensitive amperometric phenol biosensor. Biosens Bioelect 23:648
Kulys J, Schmid RD (1990) A sensitive enzyme electrode for phenol monitoring. Anal Lett 23(4):589
Coche-Guérente L, Desprez V, Labbé P, Therias S (1999) Amplification of amperometric biosensor responses by electrochemical substrate recycling. Part II: Experimental study of the catechol-polyphenol oxidase system immobilized in a laponite clay matrix. J Electroanal Chem 470:61
Kulys JJ, Vidziunaite RA (2003) Amperometric biosensors based on recombinant laccases for phenols determination. Biosens Bioelectron 319:18
Britz D (1988) Digital simulation in electrochemistry, 2nd ed. Springer, Berlin
Bartlett PN, Pratt KFE (1993) Modelling of process in enzyme electrodes. Biosens Bioelectron 8:451
Bacha S, Bergel A, Comtat M (1995) Transient response of multilayer electroenzymatic biosensors. Anal Chem 1669:67
Bacha S, Montagné M, Bergel A (1996) Modelling mass transfer with enzymatic reaction in electrochemical multilayer microreactors. Aiche J 2967:42, n°10
Coche-Guérente L, Desprez V, Labbé P, Therias S (1999) Amplification of amperometric biosensor responses by electrochemical substrate recycling. Part I: Theoretical treatment of the catechol-polyphenol oxidase system. J Electroanal Chem 470:53
Baronas R, Kulys J, Ivanauskas F (2004) Modelling amperometric enzyme electrode with substrate cyclic conversion. Biosens Bioelectron 19:915
Nowinski SA, Anjo DM (1989) Determination of the diffusion coefficients of catecholamines by potential step chronoamperometry. J Chem Eng Data 265:34
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourouina, M., Ourari, A. & Bourouina-bacha, S. The effect of membrane permeability on the response of a catechol biosensor. Microchim Acta 163, 171–178 (2008). https://doi.org/10.1007/s00604-008-0010-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0010-9