Abstract
Purpose
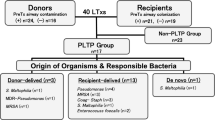
To elucidate the clinical impact of pathogenic organism (PO) positivity early after transplantation, we evaluated the impact of perioperative airway POs on outcomes after living-donor lobar lung transplantation (LDLLT), where the graft airway is supposed to be sterile from a healthy donor.
Method
A retrospective review of 67 adult LDLLT procedures involving 132 living donors was performed. Presence of POs in the recipients’ airways was evaluated preoperatively and postoperatively in intensive-care units.
Results
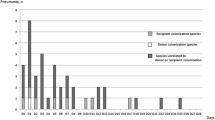
POs were detected preoperatively in 13 (19.4%) recipients. No POs were isolated from the donor airways at transplantation. POs were detected in 39 (58.2%) recipients postoperatively; most were different from the POs isolated preoperatively. Postoperative PO isolation was not associated with short-term outcomes other than prolonged postoperative ventilation. The 5-year overall survival was significantly better in the PO-negative group than in the PO-positive group (89.1% vs. 63.7%, P = 0.014). In the multivariate analysis, advanced age (hazard ratio [HR]: 1.041 per 1-year increase, P = 0.033) and posttransplant PO positivity in the airway (HR: 3.684, P = 0.019) significantly affected the survival.
Conclusions
The airways of the living-donor grafts were microbiologically sterile. PO positivity in the airway early after transplantation negatively impacted long-term outcomes.



Similar content being viewed by others
Abbreviations
- Abx:
-
Antibiotic drug
- BMI:
-
Body mass index
- BOS:
-
Bronchiolitis obliterans syndrome
- CI:
-
Confidence interval
- CLAD:
-
Chronic lung allograft dysfunction
- DDLT:
-
Deceased-donor lung transplantation
- HR:
-
Hazard ratio
- ICU:
-
Intensive-care unit
- IVIG:
-
Intravenous immunoglobulin
- LDLLT:
-
Living-donor lobar lung transplantation
- LOS:
-
Length of stay
- OS:
-
Overall survival
- PO:
-
Pathogenic organism
- POD:
-
Postoperative day
- PTLD:
-
Posttransplant lymphoproliferative disorders
- RAS:
-
Restrictive allograft syndrome
References
Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, et al. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35(10):1170–84.
Tanaka S, Geneve C, Tebano G, Grall N, Piednoir P, Bronchard R, et al. Morbidity and mortality related to pneumonia and TRACHEOBRONCHITIS in ICU after lung transplantation. BMC Pulm Med. 2018;18(1):43.
Tanaka S, Gauthier JM, Terada Y, Takahashi T, Li W, Hashimoto K, et al. Bacterial products in donor airways prevent the induction of lung transplant tolerance. Am J Transplant. 2021;21(1):353–61.
Weill D, Dey GC, Hicks RA, Young KR Jr, Zorn GL Jr, Kirklin JK, et al. A positive donor gram stain does not predict outcome following lung transplantation. J Heart Lung Transplant. 2002;21(5):555–8.
Tanaka S, Kayawake H, Yamada Y, Yutaka Y, Ohsumi A, Nakajima D, et al. Outcome after lung transplantation from a donor with bacterial pneumonia under the Japanese donor evaluation system. Transplant Proc. 2022;54(3):782–8.
Valentine VG, Gupta MR, Walker JE Jr, Seoane L, Bonvillain RW, Lombard GA, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2009;28(2):163–9.
Howell CK, Paciullo CA, Lyon GM, Neujahr D, Lyu P, Cotsonis G, et al. Effect of positive perioperative donor and recipient respiratory bacterial cultures on early post-transplant outcomes in lung transplant recipients. Transpl Infect Dis. 2017. https://doi.org/10.1111/tid.12760.
Date H. Living-related lung transplantation. J Thorac Dis. 2017;9(9):3362–71.
Nakajima D, Date H. Living-donor lobar lung transplantation. J Thorac Dis. 2021;13(11):6594–601.
Date H, Sato M, Aoyama A, Yamada T, Mizota T, Kinoshita H, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients†. Eur J Cardiothorac Surg. 2015;47(6):967–73.
Date H, Aoe M, Nagahiro I, Sano Y, Andou A, Matsubara H, et al. Living-donor lobar lung transplantation for various lung diseases. J Thorac Cardiovasc Surg. 2003;126(2):476–81.
Ikeda M, Bando T, Yamada T, Sato M, Menjyu T, Aoyama A, et al. Clinical application of ET-Kyoto solution for lung transplantation. Surg Today. 2015;45(4):439–43.
Miyoshi R, Chen-Yoshikawa TF, Hamaji M, Kawaguchi A, Kayawake H, Hijiya K, et al. Effect of early tracheostomy on clinical outcomes in critically ill lung transplant recipients. Gen Thorac Cardiovasc Surg. 2018;66(9):529–36.
Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-a consensus report from the pulmonary council of the ISHLT. J Heart Lung Transplant. 2019;38(5):493–503.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
McAnally KJ, Valentine VG, LaPlace SG, McFadden PM, Seoane L, Taylor DE. Effect of pre-transplantation prednisone on survival after lung transplantation. J Heart Lung Transplant. 2006;25(1):67–74.
Sugimoto S, Miyoshi K, Kurosaki T, Otani S, Yamane M, Kobayashi M, et al. Favorable survival in lung transplant recipients on preoperative low-dose, as compared to high-dose corticosteroids, after hematopoietic stem cell transplantation. Int J Hematol. 2018;107(6):696–702.
Horvath J, Dummer S, Loyd J, Walker B, Merrill WH, Frist WH. Infection in the transplanted and native lung after single lung transplantation. Chest. 1993;104(3):681–5.
Vos R, Blondeau K, Vanaudenaerde BM, Mertens V, Van Raemdonck DE, Sifrim D, et al. Airway colonization and gastric aspiration after lung transplantation: do birds of a feather flock together? J Heart Lung Transplant. 2008;27(8):843–9.
Walter S, Gudowius P, Bosshammer J, Römling U, Weissbrodt H, Schürmann W, et al. Epidemiology of chronic Pseudomonas aeruginosa infections in the airways of lung transplant recipients with cystic fibrosis. Thorax. 1997;52(4):318–21.
Kariya S, Okano M, Oto T, Higaki T, Haruna T, Noda Y, et al. The impact of chronic rhinosinusitis on long-term survival in lung transplantation recipients. Acta Otolaryngol. 2017;137(5):529–33.
Dowling RD, Williams P, Zenati M, Griffith BP, Hardesty RL. Infections and pathologic factors in the donor lung. J Thorac Cardiovasc Surg. 1995;109(6):1263–4.
Suryadinata R, Levin K, Holsworth L, Paraskeva M, Robinson P. Airway cilia recovery post lung transplantation. Immun Inflamm Dis. 2021;9(4):1716–23.
Duarte AG, Terminella L, Smith JT, Myers AC, Campbell G, Lick S. Restoration of cough reflex in lung transplant recipients. Chest. 2008;134(2):310–6.
Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE. 2011;6(2): e16384.
Flanagan JL, Brodie EL, Weng L, Lynch SV, Garcia O, Brown R, et al. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. J Clin Microbiol. 2007;45(6):1954–62.
Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci U S A. 2007;104(51):20529–33.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5(1): e8578.
Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186(6):536–45.
Saito M, Chen-Yoshikawa TF, Nakamoto Y, Kayawake H, Tokuno J, Ueda S, et al. Unilateral chronic lung allograft dysfunction assessed by biphasic computed tomographic volumetry in bilateral living-donor lobar lung transplantation. Transplant Direct. 2018;4(11): e398.
Sugimoto S, Yamamoto H, Kurosaki T, Otani S, Okazaki M, Yamane M, et al. Impact of chronic lung allograft dysfunction, especially restrictive allograft syndrome, on the survival after living-donor lobar lung transplantation compared with cadaveric lung transplantation in adults: a single-center experience. Surg Today. 2019;49(8):686–93.
Baumann B, Byers S, Wasserman-Wincko T, Smith L, Hathaway B, Bhama J, et al. Postoperative swallowing assessment after lung transplantation. Ann Thorac Surg. 2017;104(1):308–12.
Sharma NS, Wille KM, Athira S, Zhi D, Hough KP, Diaz-Guzman E, et al. Distal airway microbiome is associated with immunoregulatory myeloid cell responses in lung transplant recipients. J Heart Lung Transplant. 2017;S1053–2498(17):31898–903.
Acknowledgements
This work was supported by the SPIRITS 2021 of Kyoto University and the Japan Society for the Promotion of Science KAKENHI (20H03769).
Funding
This work was supported by SPIRITS 2021 of Kyoto University and Japan Society for the Promotion of Science KAKENHI (20H03769).
Author information
Authors and Affiliations
Contributions
HO conceived the primary hypothesis, designed the research, collected and analyzed the data, and wrote the manuscript. ST conceived the primary hypothesis, designed the research, analyzed the data, and wrote the manuscript. TFCY conceived the primary hypothesis, designed the research, and wrote the manuscript. Y. collected and analyzed the data and wrote the manuscript. YY, YY, DN, MH, AO, and TM contributed to the methodology and edited the manuscript. MN analyzed the data, contributed to the methodology, and edited the manuscript. HD supervised the research and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No authors have any conflicts of interest related to this work.
Informed patient consent/consent to publication
This study was approved by the Institutional Review Board of the Kyoto University Hospital (R2389), and informed consent was waived because of the retrospective nature of the study. Opt-out was not requested from any patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oda, H., Tanaka, S., Chen-Yoshikawa, T.F. et al. Impact of perioperative airway pathogens on living-donor lobar lung transplantation outcomes. Surg Today 54, 266–274 (2024). https://doi.org/10.1007/s00595-023-02730-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02730-9