Abstract
Purpose
Adherence to oral nutritional supplements (ONS) to prevent weight loss after gastrectomy is problematic. The present study evaluated the impact of super energy-dense ONS (SED ONS; 4 kcal/mL) on glycemic change and energy intake after gastrectomy.
Methods
Gastrectomy patients were placed on continuous glucose monitoring for a 3-day observation period after food intake had been stabilized postoperatively. In addition, they were given 0, 200, and 400 kcal/day of SED ONS on Days 1, 2, and 3, respectively. The primary outcome was the area under the curve < glucose 70 mg/dL (AUC < 70). The secondary outcomes were other indices of glucose fluctuation and the amount of food and SED ONS intake.
Results
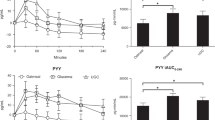
Seventeen patients were enrolled. The AUC < 70 did not differ significantly with or without SED ONS over the observation period. SED ONS did not cause postprandial hypoglycemia and prevented nocturnal hypoglycemia. The mean dietary intake did not change significantly during the observation period, and the total energy intake increased significantly according to the amount of SED ONS provided.
Conclusion
SED ONS after gastrectomy increased the total energy intake without dietary reduction and it did not result in hypoglycemia.






Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):21.
Tsuburaya A, Noguchi Y, Yoshikawa T, Nomura K, Fukuzawa K, Makino T, et al. Long-term effect of radical gastrectomy on nutrition and immunity. Surg Today. 1993;23:320–4.
Wu CW, Hsieh MC, Lo SS, Lui WY, P’Eng FK. Quality of life of patients with gastric adenocarcinoma after curative gastrectomy. World J Surg. 1997;21:777–82.
Hatao F, Chen KY, Wu JM, Wang MY, Aikou S, Onoyama H, et al. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer. Langenbecks Arch Surg. 2017;402:203–11.
Imamura H, Nishikawa K, Kishi K, Inoue K, Matsuyama J, Akamaru Y, et al. Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol. 2016;23:2928–35.
Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr. 2021;40:40–6.
Ida S, Hiki N, Cho H, Sakamaki K, Ito S, Fujitani K, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. 2017;104:377–83.
Kong SH, Lee HJ, Na JR, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery. 2018;164:1263–70.
Akashi T, Hashimoto R, Funakoshi A. Effect of a novel, energy-dense, low-volume nutritional food in the treatment of superior mesenteric artery syndrome. Cureus. 2021;13: e15243.
Holdsworth CD, Turner D, McIntyre N. Pathophysiology of post-gastrectomy hypoglycemia. Br Med J. 1969;4:257–9.
International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385–96.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603.
Kubota T, Shoda K, Ushigome E, Kosuga T, Konishi H, Shiozaki A, et al. Utility of continuous glucose monitoring following gastrectomy. Gastric Cancer. 2020;23:699–706.
Ri M, Nunobe S, Ida S, Ishizuka N, Atsumi S, Makuuchi R, et al. Preliminary prospective study of real-time post-gastrectomy glycemic fluctuations during dumping symptoms using continuous glucose monitoring. World J Gastroenterol. 2021;27:3386–95.
Ri M, Nunobe S, Ida S, Ishizuka N, Atsumi S, Hatami M, et al. Postprandial asymptomatic glycemic fluctuations after gastrectomy for gastric cancer using continuous glucose monitoring device. J Gastric Cancer. 2021;21:325–34.
Shivaprasad C, Aiswarya Y, Kejal S, Sridevi A, Anupam B, Ramdas B, et al. Comparison of CGM-derived measures of glycemic variability between pancreatogenic diabetes and type 2 diabetes mellitus. J Diabetes Sci Technol. 2021;15:134–40.
Iwasaki S, Kozawa J, Kimura T, Fukui K, Iwahashi H, Imagawa A, et al. Insulin degludec is associated with less frequent and milder hypoglycemia in insulin-deficient patients with type 1 diabetes compared with insulin glargine or detemir. Diabetol Int. 2017;8:228–36.
Matsumura M, Nakatani Y, Tanka S, Aoki C, Sagara M, Yanagi K, et al. Efficacy of additional canagliflozin administration to type 2 diabetes patients receiving insulin therapy: examination of diurnal glycemic patterns using continuous glucose monitoring (CGM). Diabetes Ther. 2017;8:821–7.
Kobayashi D, Ishigure K, Mochizuki Y, et al. Multi-institutional prospective feasibility study to explore tolerability and efficacy of oral nutritional supplements for patients with gastric cancer undergoing gastrectomy (CCOG1301). Gastric Cancer. 2017;20:718–27.
Miyazaki Y, Omori T, Fujitani K, Fujita J, Kawabata R, Imamura H, et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer. 2021;24:1150–9.
Scarpellini E, Arts J, Karamanolis G, Laurenius A, Siquini W, Suzuki H, et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16:448–66.
Shimizu N, Oki E, Tanizawa Y, Suzuki Y, Aikou S, Kunisaki C, et al. Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: a Japanese multicenter, randomized controlled trial. Surg Today. 2018;48:865–74.
Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8.
Nakajima K, Mita T, Osonoi Y, Azuma K, Takasu T, Fujitani Y, et al. Effect of repetitive glucose spike and hypoglycaemia on atherosclerosis and death rate in apo E-deficient mice. Int J Endocrinol. 2015;2015: 406394.
Whitmer RA, Karter AJ, Yafe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–72.
Mehta HB, Mehta V, Goodwin JS. Association of hypoglycemia with subsequent dementia in older patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci. 2017;72:1110–6.
Krakauer M, Botero JF, Lavalle-González FJ, Proietti A, Barbieri DE. A review of flash glucose monitoring in type 2 diabetes. Diabetol Metab Syndr. 2021;13:42.
Yajima T, Takahashi H, Yasuda K. Comparison of interstitial fluid glucose levels obtained by continuous glucose monitoring and flash glucose monitoring in patients with type 2 diabetes mellitus undergoing hemodialysis. J Diabetes Sci Technol. 2020;14:1088–94.
Hásková A, Radovnická L, Petruželková L, Parkin CG, Grunberger G, Horová E, et al. Real-time cgm is superior to flash glucose monitoring for glucose control in type 1 diabetes: the corrida randomized controlled trial. Diabetes Care. 2020;43:2744–50.
Acknowledgements
We thank Mr. James R. Valera for his assistance with editing this manuscript and Mr. Atsushi Nishida, Mr. Syudo Yamasaki, and Mr. Satoshi Usami for their assistance with the statistical analysis. We are especially grateful to Ms. Tokiko Matsukura, Ms. Keiko Yokota, and the other dietitians at our center for providing nutrition education and evaluating the patients’ caloric intake.
Funding
The present study was supported by a grant from the Clinical Research Fund of the Tokyo Metropolitan Government (Grant no. R02050302).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Ethical approval
All data collection and analysis were performed in accordance with the ethical standards of the Declaration of Helsinki. This study was approved by the ethics committee of Tokyo Metropolitan Tama Medical Center (No.3–6) and registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR 000041219).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamazaki, R., Hatao, F., Itokawa, M. et al. Impact of super energy-dense oral nutritional supplementation (SED ONS) on glycemic variability and food intake postoperatively in gastric cancer patients. Surg Today 53, 605–613 (2023). https://doi.org/10.1007/s00595-022-02600-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02600-w