Abstract
Background and objectives
Tyrosine kinase inhibitors (TKIs) have provided excellent clinical benefits to patients with advanced differentiated thyroid cancer (DTC): however, the tumor status for which maximum efficacy can be obtained remains controversial. We conducted this study to identify effective clinical predictors, focusing on disease progression.
Methods
Using the data of 42 DTC patients treated with lenvatinib, we investigated the clinical factors related to overall survival (OS) and progression-free survival (PFS), and conducted analyses by the scoring of the factors.
Results
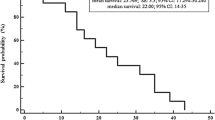
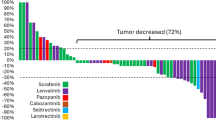
The 3 year OS and median PFS were 51% and 13.8 months, respectively. Univariate analysis identified performance status (PS), tumor-related symptoms, and tumor diameter as the only factors affecting both these outcomes. Giving 1-point for each of these three factors, a higher score was significantly related to shorter OS and PFS. Patients with two or fewer points (n = 34) had better median OS (NR vs 3.9 months, p < 0.001) and PFS (15.7 vs 2.1 months, p < 0.001) than patients with three points (n = 8). Patients with all three factors had a significantly worse prognosis than patients with two or fewer factors.
Conclusion
DTC patients with all three indicators are unlikely to have longer survival. Therefore, it is important to commence TKIs before disease progression.




Similar content being viewed by others
References
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30.
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28.
Berdelou A, Borget I, Godbert Y, Nguyen T, Garcia ME, et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. 2018;28(1):72−8.
Sugino K, Nagahama M, Kitagawa W, Ohkuwa K, Uruno T, et al. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr J. 2018;65:299–306.
Suzuki C, Kiyota N, Imamura Y, Goto H, Suto H, et al. Exploratory analysis of prognostic factors for lenvatinib in radioiodine-refractory differentiated thyroid cancer. Head Neck. 2019;41:3023–32.
Fukuda N, Takahashi S. Clinical indications for treatment with multi-kinase inhibitors in patients with radioiodine-refractory differentiated thyroid cancer. Cancers (Basel). 2021;13:2279–96.
Masaki C, Sugino K, Saito N, Akaishi J, Hames KY, et al. Efficacy and limitations of lenvatinib therapy for radioiodine-refractory differentiated thyroid cancer: real-world experiences. Thyroid. 2020;30:214–21.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
National comprehensive cancer Network clinical Practice Guidelines in Oncology Thyroid carcinoma. 2019. Version 2, (2019) NCCN Guidelines.
Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, et al. 2019 European thyroid association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur Thyroid J. 2019;8:227–45.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21:707–16.
Sabra MM, Ghossein R, Tuttle RM. Time course and predictors of structural disease progression in pulmonary metastases arising from follicular cell-derived thyroid cancer. Thyroid. 2016;26:518–24.
Sabra MM, Sherman EJ, Tuttle RM. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer. 2017;123:2955–64.
Tuttle RM, Brose MS, Grande E, Kim SW, Tahara M, et al. Novel concepts for initiating multitargeted kinase inhibitors in radioactive iodine refractory differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017;31:295–305.
Masaki C, Sugino K, Saito N, Saito Y, Tanaka T, et al. Lenvatinib induces early tumor shrinkage in patients with advanced thyroid carcinoma. Endocr J. 2017;64:819–26.
Brulde B. The goals of medicine. Towards a unified theory. Health Care Anal. 2001;9:1–13.
Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J Clin Oncol. 2017;35:2692–9.
Elisei R ea, (2015) Subgroup analysis according to differentiated thyroid cancer histology in the phase 3 (SELECT) trial of lenvatinib.
Wirth L ea, ( 2019) Influence of tumor size and eastern cooperative oncology group performance status at baseline on patient outcomes in lenvatinib-treated radioiodine-refractory differentiated Thyroid cancer. . Program of the American Society of Clinical Oncology Annual Meeting 2019, Chicago, Illinois, USA, Poster No. 6081.
Robinson B, Schlumberger M, Wirth LJ, Dutcus CE, Song J, et al. Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101:4103–9.
Tahara M, Kiyota N, Hoff AO, Badiu C, Owonikoko TK, et al. Impact of lung metastases on overall survival in the phase 3 SELECT study of lenvatinib in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer. 2021;147:51–7.
Tahara M, Schlumberger M, Elisei R, Habra MA, Kiyota N, et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur J Cancer. 2017;75:213–21.
Wirth L ea, ( 2019) Lenvatinib (LEN) in Patients With Radioiodine-Refractory Differentiated Thyroid Cancer (RR-DTC) in SELECT: Post Hoc Analysis of Neutrophil-to-Lymphocyte Ratio (NLR). Program of the World Congress on Thyroid Cancer 35, Rome, Italy, Oral Paper Session #7–2.
Gianoukakis AG, Dutcus CE, Batty N, Guo M, Baig M. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer. 2018;25:699–704.
Haddad RI, Schlumberger M, Wirth LJ, Sherman EJ, Shah MH, et al. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017;56:121–8.
Tahara M, Brose MS, Wirth LJ, Suzuki T, Miyagishi H, et al. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer. 2019;106:61–8.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
Feng J, Wang Y, Shan G, Gao L. Clinical and prognostic value of neutrophil-lymphocyte ratio for patients with thyroid cancer: a meta-analysis. Medicine (Baltimore). 2020;99:19686.
Fukuda N, Wang X, Ohmoto A, Urasaki T, Sato Y, et al. Sequential analysis of neutrophil-to-lymphocyte ratio for differentiated thyroid cancer patients treated with lenvatinib. In Vivo. 2020;34:709–14.
Ito Y, Onoda N, Kihara M, Miya A, Miyauchi A. Prognostic significance of neutrophil-to-lymphocyte ratio in differentiated thyroid carcinoma having distant metastasis: a comparison with thyroglobulin-doubling rate and tumor volume-doubling rate. In Vivo. 2021;35:1125–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masaki, C., Sugino, K., Saito, N. et al. Predictors of maximum efficacy of lenvatinib for real-world patients with differentiated thyroid carcinoma. Surg Today 52, 1660–1669 (2022). https://doi.org/10.1007/s00595-022-02498-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02498-4