Abstract
Purpose
Acute peritonitis has remained a fatal disease despite of recent advances in care and treatment, including antibiotic and anticoagulant treatments. The cause of death is mostly sepsis-induced multiple organ failure. Oxidative stress can play an important role in this situation, but antioxidant therapy to capture any excessive reactive oxygen species has not yet been fully established.
Methods
Two experiments were performed. In the first experiment, we confirmed the effects of peritoneal lavage with hydrogen-rich saline (HRS) after a cecal ligation and puncture (CLP) operation in rats. In the second experiment, the changes in the hemodynamic state following this procedure were observed in a porcine model of abdominal sepsis to evaluate its safety and utility.
Results
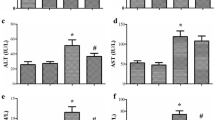
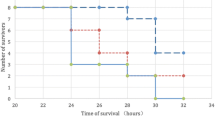
Peritoneal lavage with HRS significantly improved the survival after CLP in rats, and it ameliorated the levels of sepsis-induced organ failure. Moreover, it showed anti-inflammatory and anti-apoptosis as well as antioxidant effects. The second experiment demonstrated the potential safety and feasibility of this procedure in a large animal model.
Conclusion
This procedure can improve survival after sepsis through mitigating the sepsis-induced organ failure by inhibiting oxidative stress, apoptosis, and inflammatory pathways. Peritoneal lavage with HRS may therefore be an effective, safe, and practical therapy for patients with acute peritonitis.












Similar content being viewed by others
References
Sartelli M, Abu-Zidan FM, Catena F, Griffiths EA, Di Saverio S, Coimbra R, et al. Global validation of the WSES sepsis severity score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study). World J Emerg Surg. 2015;10:61.
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54.
Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Integr Comp Physiol. 2004;286:R491–7.
Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–77.
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74.
Zheng GJ, Sun Q, Li YP. Inflammation, endothelium, coagulation in sepsis. Chin Crit Care Med. 2009;21:573–6.
Nimah M, Brilli RJ. Coagulation dysfunction in sepsis and multiple organ system failure. Crit Care Clin. 2003;19:441–58.
Niederwanger C, Hell T, Hofer S, Salvador C, Michel M, Schenk B, et al. Antithrombin deficiency is associated with mortality and impaired organ function in septic pediatric patients: a retrospective study. PeerJ. 2018;6:e5538.
Gando S, Saitoh D, Ishikura H, Ueyama M, Otomo Y, Oda S, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17:R297.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–77.
Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23.
Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–41.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Ozawa T. Mitochondrial genome mutation in cell death and aging. J Bioenerg Biomembr. 1999;31:377–90.
Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013;110:472–80.
Oliveira GP, Oliveira MBG, Santos RS, Lima LD, Dias CM, Saber AMAB, et al. Intravenous glutamine decreases lung and distal organ injury in an experimental model of abdominal sepsis. Crit Care. 2009;13:R74.
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94.
Zhang J, Wu Q, Song S, Wan Y, Zhang R, Tai M, et al. Effect of hydrogen-rich water on acute peritonitis of rat models. Int Immunopharmacol. 2014;21:94–101.
Xie K, Liu L, Yu Y, Wang G. Hydrogen gas presents a promising therapeutic strategy for sepsis. Biomed Res Int. 2014;2014:807635.
Li GM, Ji MH, Sun XJ, Zeng QT, Tian M, Fan YX, et al. Effects of hydrogen-rich saline treatment on polymicrobial sepsis. J Surg Res. 2013;181:279–86.
Schein M, Gecelter G, Freinkel W, Gerding H, Becker PJ. Peritoneal lavage in abdominal sepsis: a controlled clinical study. Arch Surg. 1990;125:1132–5.
Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201.
Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, et al. Cecal ligation and puncture. Shock. 2005;24:52–7.
Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA. 1988;85:6465–7.
Li J, Hong Z, Liu H, Zhou J, Cui L, Yuan S, et al. Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front Pharmacol. 2016;7:106.
Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–33.
Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–9.
Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol. 2010;185:569–77.
Biswal SRD. Sepsis: redox mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2007;9:1959–61.
Garrabou G, Morén C, López S, Tobías E, Cardellach F, Miró Ò, et al. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400.
Huang LJ, Hsu C, Tsai TN, Wang SJ, Yang RC. Suppression of mitochondrial ATPase inhibitor protein (IF1) in the liver of late septic rats. Biochim Biophys Acta-Bioenerg. 2007;1767:888–96.
Terawaki H, Zhu WJ, Matsuyama Y, Terada T, Takahashi Y, Sakurai K, et al. Effect of a hydrogen (H2)-enriched solution on the albumin redox of hemodialysis patients. Hemodial Int. 2014;18:459–66.
Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836–9.
Terawaki H, Hayashi Y, Zhu W-J, Matsuyama Y, Terada T, Kabayama S, et al. Transperitoneal administration of dissolved hydrogen for peritoneal dialysis patients: a novel approach to suppress oxidative stress in the peritoneal cavity. Med Gas Res. 2013;3:14.
Song G, Li M, Sang H, Zhang L, Li X, Yao S, et al. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res. 2013;54(7):1884–93.
Nagatani K, Nawashiro H, Takeuchi S, Tomura S, Otani N, Osada H, et al. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: initial clinical studies. Med Gas Res. 2013;3:13.
Matsumoto S, Ueda T, Kakizaki H. Effect of supplementation with hydrogen-rich water in patients with interstitial cystitis/painful bladder syndrome. Urology. 2013;81:226–30.
Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Kaneko K, et al. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Med Gas Res. 2012;2:21.
Chen Y, Zong C, Guo Y, Tian L. Hydrogen-rich saline may be an effective and specific novel treatment for osteoradionecrosis of the jaw. Ther Clin Risk Manag. 2015;11:1581–5.
Gao Y, Yang H, Fan Y, Li L, Fang J, Yang W. Hydrogen-rich saline attenuates cardiac and hepatic injury in doxorubicin rat model by inhibiting inflammation and apoptosis. Mediat Inflamm. 2016;206:1–10.
Takahashi M, Chen-Yoshikawa TF, Saito M, Tanaka S, Miyamoto E, Ohata K, et al. Immersing lungs in hydrogen-rich saline attenuates lung ischaemia-reperfusion injury. Eur J Cardio-thoracic Surg. 2017;51:442–8.
Acknowledgements
This work was carried out at the Research Facilities for Laboratory Animal Science, Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Haruki Sada, Hiroyuki Egi, Kentaro Ide, Hiroyuki Sawada, Yusuke Sumi, Minoru Hattori, Kazuhiro Sentani, Naohide Oue, Wataru Yasui and Hideki Ohdan declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of institution or practice at which the studies were conducted. All experiments using rats were performed under protocols approved by the Institutional Animal Care and Use Committee of Hiroshima University (number: A14-133-4) and those for pigs were approved by the Animal Care and Use Committee of Intervention Technical Center (IVTeC), Japan (number: IVT 16-10).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sada, H., Egi, H., Ide, K. et al. Peritoneal lavage with hydrogen-rich saline can be an effective and practical procedure for acute peritonitis. Surg Today 51, 1860–1871 (2021). https://doi.org/10.1007/s00595-021-02271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02271-z