Abstract
Purpose
Papillary thyroid cancer (PTC) is generally associated with a favorable prognosis. However, some patients have fatal disease, with locally infiltrating tumors or progressive distant metastases; yet few studies have investigated the characteristics of the tumor-progressive gene expression profile in advanced PTC. We conducted this study to clarify the gene expression status in advanced PTC and identify candidate molecules for prognostic biomarkers.
Methods
We analyzed 740 tumor-progressive gene expression levels from formalin-fixed paraffin-embedded blocks of samples from six patients with low-risk PTC and six patients with high-risk PTC, using the nCounter PanCancer Progression panel. Then, we investigated the association between the expression levels of focused genes and pathological factors in PTC patients in The Cancer Genome Atlas (TCGA) database.
Results
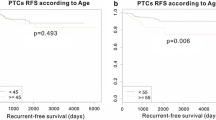
The expression levels of 14 genes in the high-risk PTC specimens were more than two-fold those in the low-risk PTC specimens. In the TCGA database, expression levels of four genes (CCL11, COL6A3, INHBA, and SRPX2) were significantly higher in patients with advanced PTC. Among the patients with advanced PTC, those with high SRPX2 expression levels had poor disease-free survival. Univariate and multivariate analyses revealed that high SRPX2 expression was an independent prognostic factor.
Conclusion
Based on the findings of this study, CCL11, COL6A3, INHBA, and SRPX2 are potential biomarkers that indicate advanced PTC. SRPX2, in particular, is considered a prognostic biomarker.



Similar content being viewed by others
References
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–7.
Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–31.
Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67:669–717.
Sugitani I, Ito Y, Miyauchi A, Imai T, Suzuki S. Active surveillance versus immediate surgery: questionnaire survey on the current treatment strategy for adult patients with low-risk papillary thyroid microcarcinoma in Japan. Thyroid. 2019;29:1563–71.
Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30:1856–83.
Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore). 2012;91:274–86.
Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–501.
Bournaud C, Descotes F, Decaussin-Petrucci M, Berthiller J, de la Fouchardiere C, Giraudet AL, et al. TERT promoter mutations identify a high-risk group in metastasis-free advanced thyroid carcinoma. Eur J Cancer. 2019;108:41–9.
Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99:E754–65.
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–46.
Tian M, Chen L, Ma L, Wang D, Shao B, Wu J, et al. Expression and prognostic significance of CCL11/CCR3 in glioblastoma. Oncotarget. 2016;7:32617–27.
Johrer K, Zelle-Rieser C, Perathoner A, Moser P, Hager M, Ramoner R, et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005;11:2459–65.
Levina V, Nolen BM, Marrangoni AM, Cheng P, Marks JR, Szczepanski MJ, et al. Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009;15:2647–56.
Svoronos C, Tsoulfas G, Souvatzi M, Chatzitheoklitos E. Prognostic value of COL6A3 in pancreatic adenocarcinoma. Ann Hepatobiliary Pancreat Surg. 2020;24:52–6.
Duan Y, Liu G, Sun Y, Wu J, Xiong Z, Jin T, et al. COL6A3 polymorphisms were associated with lung cancer risk in a Chinese population. Respir Res. 2019;20:143.
Liu W, Li L, Ye H, Tao H, He H. Role of COL6A3 in colorectal cancer. Oncol Rep. 2018;39:2527–36.
Xie X, Liu X, Zhang Q, Yu J. Overexpression of collagen VI alpha3 in gastric cancer. Oncol Lett. 2014;7:1537–43.
Li X, Yang Z, Xu S, Wang Z, Jin P, Yang X, et al. Targeting INHBA in ovarian cancer cells suppresses cancer xenograft growth by attenuating stromal fibroblast activation. Dis Markers. 2019;2019:7275289.
Li X, Yu W, Liang C, Xu Y, Zhang M, Ding X, et al. INHBA is a prognostic predictor for patients with colon adenocarcinoma. BMC Cancer. 2020;20:305.
He F, Wang H, Li Y, Liu W, Gao X, Chen D, et al. SRPX2 knockdown inhibits cell proliferation and metastasis and promotes chemosensitivity in esophageal squamous cell carcinoma. Biomed Pharmacother. 2019;109:671–8.
Tanaka K, Arao T, Maegawa M, Matsumoto K, Kaneda H, Kudo K, et al. SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int J Cancer. 2009;124:1072–80.
Yang S, Shin J, Park KH, Jeung HC, Rha SY, Noh SH, et al. Molecular basis of the differences between normal and tumor tissues of gastric cancer. Biochim Biophys Acta. 2007;1772:1033–40.
Hong X, Zhao H, He C. Knockdown of SRPX2 inhibits the proliferation, migration, and invasion of prostate cancer cells through the PI3K/Akt/mTOR signaling pathway. J Biochem Mol Toxicol. 2018;2018:22237.
Ao R, Guan L, Wang Y, Wang JN. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-Akt signaling pathway. J Cell Biochem. 2018;119:4420–34.
Shibata M, Ham K, Hoque MO. A time for YAP1: Tumorigenesis, immunosuppression and targeted therapy. Int J Cancer. 2018;143:2133–44.
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28.
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We do not have any potential conflicts of interest associated with this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
595_2021_2262_MOESM1_ESM.pptx
Supplementary Fig. 1 Association between gene expression levels and pathological stages in The Cancer Genome Atlas (TCGA) database. None of the 11 genes other than CCL11, COL6A3, INHBA, and SRPX2, showed significantly higher expressions in patients with pT4 and pN1. *p < 0.05, ****p < 0.0001, N. S.: not significant (PPTX 160 KB)
Rights and permissions
About this article
Cite this article
Shibata, M., Inaishi, T., Ichikawa, T. et al. Identifying the tumor-progressive gene expression profile in high-risk papillary thyroid cancer. Surg Today 51, 1703–1712 (2021). https://doi.org/10.1007/s00595-021-02262-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02262-0