Abstract
Purpose
To propose a new and improved surveillance schedule for colorectal cancer (CRC) patients by focusing on the recurrence rate, resectability, and especially, the tumor doubling time (DT) of recurrent tumors.
Methods
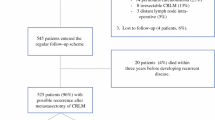
The subjects of this retrospective review were 1774 consecutive patients who underwent radical surgery for stage I–III CRC between January, 2004 and December, 2015. We calculated the DT by measuring the tumor diameter using computed tomography (CT).
Results
The median DT for recurrences in the liver, lung, peritoneum, and other locations were 35, 72, 85, and 36 days, respectively, (p < 0.001) and tumor growth rates differed according to the organs where recurrence developed. Multiple linear regression analysis showed that the DT was strongly associated with the relapse-free interval from primary surgery (p < 0.001), and that the DT in patients with recurrence detected ≥ 3 years after primary surgery was longer by 151.1 days than that in patients with recurrence detected within 1 year after primary surgery. We proposed a less intensive surveillance, which achieved an average cost reduction of 32.5% compared with conventional surveillance in Japan.
Conclusion
We propose a new and more cost-efficient surveillance schedule for CRC surgery patients in the clinical setting.


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003;50:1362–6.
Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. https://doi.org/10.1097/01.sla.0000128305.90650.71.
Ike H, Shimada H, Ohki S, Togo S, Yamaguchi S, Ichikawa Y. Results of aggressive resection of lung metastases from colorectal carcinoma detected by intensive follow-up. Dis Colon Rectum. 2002;45:468–73. https://doi.org/10.1007/s10350-004-6222-0.
Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–75. https://doi.org/10.1016/j.jamcollsurg.2005.11.008.
Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2002;1:002200. https://doi.org/10.1002/14651858.CD002200.
Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. https://doi.org/10.1186/1471-2407-3-26.
Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–99. https://doi.org/10.1007/s10350-007-9030-5.
Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. https://doi.org/10.1136/bmj.324.7341.813.
Wille-Jorgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, et al. Effect of more vs. less frequent follow-up testing on overall and colorectal cancer-specific mortality in patients with stage ii or iii colorectal cancer: the COLOFOL randomized clinical trial. JAMA. 2018;319:2095–103. https://doi.org/10.1001/jama.2018.5623.
Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:002200. https://doi.org/10.1002/14651858.CD002200.pub3.
Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–70. https://doi.org/10.1001/jama.2013.285718.
Mant D, Gray A, Pugh S, Campbell H, George S, Fuller A, et al. A randomised controlled trial to assess the cost-effectiveness of intensive versus no scheduled follow-up in patients who have undergone resection for colorectal cancer with curative intent. Health Technol Assess. 2017;21:1–86. https://doi.org/10.3310/hta21320.
Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol. 2016;27:274–80. https://doi.org/10.1093/annonc/mdv541.
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. https://doi.org/10.1007/s10147-017-1101-6.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology-colon cancer, version 2. 2019. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 13 Dec 2019.
Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58:713–25. https://doi.org/10.1097/DCR.0000000000000410.
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72. https://doi.org/10.1093/annonc/mdt354.
Shida D, Yoshida T, Tanabe T, Tsukamoto S, Ochiai H, Kanemitsu Y. Prognostic Impact of R0 resection and targeted therapy for colorectal cancer with synchronous peritoneal metastasis. Ann Surg Oncol. 2018;25:1646–53. https://doi.org/10.1245/s10434-018-6436-3.
Collins VP, Loeffler RK, Tivey H. Observations on growth rates of human tumors. Am J Roentgenol Radium Ther Nucl Med. 1956;76:988–1000.
Collins VP. Time of occurrence of pulmonary metastasis from carcinoma of colon and rectum. Cancer. 1962;15:387–95.
Miyake H, Murono K, Nagata H, Nozawa H, Kawai K, Hata K, et al. Prognostic significance of doubling time in patients undergoing radical surgery for metachronous peritoneal metastases of colorectal cancer. Int J Colorectal Dis. 2019;34:801–9. https://doi.org/10.1007/s00384-019-03259-5.
Tanaka K, Shimada H, Miura M, Fujii Y, Yamaguchi S, Endo I, et al. Metastatic tumor doubling time: most important prehepatectomy predictor of survival and nonrecurrence of hepatic colorectal cancer metastasis. World J Surg. 2004;28:263–70. https://doi.org/10.1007/s00268-003-7088-3.
Tanaka K, Shimada H, Fujii Y, Endo I, Sekido H, Togo S, et al. Pre-hepatectomy prognostic staging to determine treatment strategy for colorectal cancer metastases to the liver. Langenbecks Arch Surg. 2004;389:371–9. https://doi.org/10.1007/s00423-004-0490-y.
Tanaka K, Shimada H, Ueda M, Matsuo K, Endo I, Togo S. Long-term characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol. 2007;14:1336–46. https://doi.org/10.1245/s10434-006-9071-3.
Kawaguchi K, Uehara K, Nakayama G, Fukui T, Fukumoto K, Nakamura S, et al. Growth rate of chemotherapy-naive lung metastasis from colorectal cancer could be a predictor of early relapse after lung resection. Int J Clin Oncol. 2016;21:329–34. https://doi.org/10.1007/s10147-015-0889-1.
Tomimaru Y, Noura S, Ohue M, Okami J, Oda K, Higashiyama M, et al. Metastatic tumor doubling time is an independent predictor of intrapulmonary recurrence after pulmonary resection of solitary pulmonary metastasis from colorectal cancer. Dig Surg. 2008;25:220–5. https://doi.org/10.1159/000140693.
Cucchetti A, Russolillo N, Johnson P, Tarchi P, Ferrero A, Cucchi M, et al. Impact of primary cancer features on behaviour of colorectal liver metastases and survival after hepatectomy. BJS Open. 2019;3:186–94. https://doi.org/10.1002/bjs5.100.
Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–73.
Mohajeri G, Hejab K, Sheikhbahaei S, Mohajeri M, Niknam N, Mohammadi A. Micrometastasis in colorectal cancer: does it affect staging? ANZ J Surg. 2018;88:E237–E241241. https://doi.org/10.1111/ans.13809.
Alsaggar M, Yao Q, Cai H, Liu D. Differential growth and responsiveness to cancer therapy of tumor cells in different environments. Clin Exp Meta. 2016;33:115–24. https://doi.org/10.1007/s10585-015-9761-y.
Algire GH, Legallais FY, Anderson BF. Vascular reactions of normal and malignant tissues in Vivo. VI. The role of hypotension in the action of components of podophyllin on transplanted sarcomas. J Natl Cancer Inst. 1954;14:879–93.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. https://doi.org/10.1056/NEJM197111182852108.
Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. https://doi.org/10.1038/35077241.
Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–94. https://doi.org/10.3748/wjg.v19.i41.6979.
Tempero MA, Williams CA, Anderson JC. The value of hepatic ultrasound and biochemical liver tests in screening for liver metastases. J Clin Oncol. 1986;4:1074–8. https://doi.org/10.1200/JCO.1986.4.7.1074.
Schneider J, Koullouros M, Mackay C, Ramsay G, Parnaby C, Stevenson L. Is Liver ultrasound useful as part of the surveillance strategy following potentially curative colorectal cancer resection? Dig Dis. 2019;37:234–8. https://doi.org/10.1159/000495114.
Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–311. https://doi.org/10.1002/jmri.22010.
Seo SI, Lim SB, Yoon YS, Kim CW, Yu CS, Kim TW, et al. Comparison of recurrence patterns between </=5 years and >5 years after curative operations in colorectal cancer patients. J Surg Oncol. 2013;108:9–13. https://doi.org/10.1002/jso.23349.
Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. https://doi.org/10.1097/SLA.0b013e3181907fd9.
Dorr NM, Bartels M, Morgul MH. Current treatment of colorectal liver metastasis as a chronic disease. Anticancer Res. 2020;40:1–7. https://doi.org/10.21873/anticanres.13921.
Goere D, Souadka A, Faron M, Cloutier AS, Viana B, Honore C, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22:2958–64. https://doi.org/10.1245/s10434-015-4387-5.
Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–53. https://doi.org/10.1038/nm0295-149.
Funding
This research was supported by the Project for Cancer Research and Therapeutic Evolution from the Japan Agency for Medical Research and Development (grant number: JP19cm0106502) and by Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (grant numbers: 17K10620, 17K10621, 17K10623, 18K07194, 19K09114 and 19K09115).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Soichiro Ishihara receives lecture fees from EA Pharma, Alfresa, Otsuka, KAKEN, Yakult, Johnson and Johnson, TAIHO, Takeda, CHUGAI, Nippon Kayaku, Pfizer, Merck, Janssen, and research funding from TAIHO, Takeda, Mitsubishi Tanabe, CHUGAI, TSUMURA, Eli Lilly, Pfizer, MIYARISAN, Merck, MOCHIDA. The other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyake, H., Kawai, K., Nozawa, H. et al. Less intensive surveillance after radical surgery for stage I–III colorectal cancer by focusing on the doubling time of recurrence. Surg Today 51, 550–560 (2021). https://doi.org/10.1007/s00595-020-02135-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02135-y