Abstract
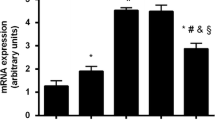
In chronically uncompensated diabetes mellitus, an increase has been observed in the content of advanced glycation endproduct (AGE)-modified proteins in various tissues, including bone. This increase can lead to a local imbalance in the secretion of cytokines and growth factors, and has been implicated in the pathophysiology of the long-term complications of diabetes. We have previously shown that the proliferation and differentiation of UMR106 rat osteosarcoma and MC3T3E1 mouse calvaria-derived cell lines are regulated by AGE-modified proteins, possibly through the recognition of these AGEs by specific membrane-associated receptors. In the present study, we investigated the effects of AGE-proteins on the secretion of insulin-like growth factor-I (IGF-I) and its binding proteins (IGFBPs) by both osteoblast-like cell lines. In the case of MC3T3E1 cells, this was studied throughout their successive stages of development: proliferation, differentiation and mineralisation. For every condition, cells were incubated 24 hours with increasing concentrations of either bovine serum albumin (BSA) or AGE-BSA. IGF-I in conditioned media was separated from IGFBPs by acid gel filtration-centrifugation, and measured by radioimmunoassay. IGFBPs in conditioned media were analysed by a semi-quantitative western ligand blot. In UMR106 cells, low doses of AGE-BSA significantly decreased the secretion of both IGF-I (56% of control) and a 24 kDa IGFBP (80% of control). Results for MC3T3E1 cells, which predominantly secrete 29 kDa IGFBPs, were dependent on the stage of development. In proliferating preosteoblastic cells, AGE-BSA decreased the secretion of IGF-I (34%–37% of control) while increasing the secretion of IGFBP (124%–127% of control). On the other hand, secretion of these components of the IGF system by mature (differentiated) cells was unaffected by the presence of AGE-BSA. When these cells finally attained mineralisation, incubation with AGE-modified BSA provoked an increase both in IGFBP (131%–169% of control) and in IGF-I secretion (119%–123% of control). The presented evidence suggests that the modulation of growth and development by AGE-modified proteins, previously described for both cell lines, could be the result of an autocrine-paracrine mechanism involving the IGF-IGFBP system.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 15 September 2000 / Accepted in revised form: 23 August 2001
Rights and permissions
About this article
Cite this article
McCarthy, A., Etcheverry, S. & Cortizo, A. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol 38, 113–122 (2001). https://doi.org/10.1007/s005920170007
Issue Date:
DOI: https://doi.org/10.1007/s005920170007