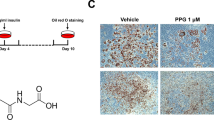
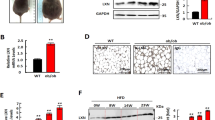
Abstract
Background
Obesity, defined as excessive or abnormal body fat accumulation, which could significantly increase the risk of cardiovascular disease, type 2 diabetes mellitus (T2DM) diseases and seriously affect people’s quality of life. More than 2 billion people are overweight, and the incidence of obesity is increasing rapidly worldwide, it has become a widely concerned public health issue in the world. Diverse evidence show that active metabolites are involved in the pathophysiological processes of obesity.
Aims
However, whether the downstream catabolite of tryptophan, 3-indole acrylic acid (IA), is involved in obesity remains unclear.
Methods
We collected the samples of serum from peripheral blood of obesity and health controls, and liquid chromatography–mass spectrometry (LC-MS) was performed to identify the plasma levels of IA. Additionally, we verified the potential benefits of IA on human preadipocytes and HFD- induced zebrafish by cell viability assay, flow cytometry assay, Oil red O staining, total cholesterol (T-CHO), triglyceride (TG) and nonesterified free fatty acids (NEFA) measurements and Nile Red staining. RNA-Seq, functional analysis and western blot revealed the mechanisms underlying the function of IA.
Results
We found that the content of IA in peripheral blood serum of overweight people was significantly lower than that of normal people. In addition, supplementation with IA in zebrafish larvae induced by a high fat diet (HFD) dramatically reduced HFD induced lipid accumulation. IA had no effect on proliferation and apoptosis of preadipocytes, but significantly inhibited adipogenesis of preadipocytes by down-regulate CEBPα and PPARγ. RNA-Seq and functional analysis revealed that IA regulated the adipogenesis of preadipocytes through stimulate the phosphorylation of STAT1.
Conclusions
Taken together, IA has been identified as a potent metabolite for the prevention or treatment of obesity.
Graphic Abstract






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available from the corresponding author upon reasonable request.
The datasets generated and analysed during the current study are available in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE246843 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE246843).
References
Lustig RH, Collier D, Kassotis C et al (2022) Obesity I: overview and molecular and biochemical mechanisms. Biochem Pharmacol 199:115012
Theilade S, Christensen MB, Vilsbøll T et al (2021) An overview of obesity mechanisms in humans: endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes Metab 23(Suppl 1):17–35
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384(9945):766–781
Prentice AM (2006) The emerging epidemic of obesity in developing countries. Int J Epidemiol 35(1):93–99
Lin X, Li H (2021) Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) 12:706978
Webb VL, Wadden TA (2017) Intensive lifestyle intervention for obesity: principles, practices, and results. Gastroenterology 152(7):1752–1764
Müller TD, Blüher M, Tschöp MH et al (2022) Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov 21(3):201–223
Gloy VL, Briel M, Bhatt DL et al (2013) Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347:f5934
Vishvanath L, Gupta RK (2019) Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest 129(10):4022–4031
Haczeyni F, Bell-Anderson KS, Farrell GC (2018) Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev 19(3):406–420
Sun K, Kusminski CM, Scherer PE (2011) Adipose tissue remodeling and obesity. J Clin Invest 121(6):2094–2101
Lefterova MI, Lazar MA (2009) New developments in adipogenesis. Trends Endocrinol Metab 20(3):107–114
Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7(12):885–896
Tang QQ, Lane MD (2012) Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 81:715–736
Haider N, Larose L (2019) Harnessing adipogenesis to prevent obesity. Adipocyte 8(1):98–104
Li VL, He Y, Contrepois K et al (2022) An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606(7915):785–790
Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fenelon VS et al (2021) Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab 33(7):1483-1492.e10
Chávez-Talavera O, Tailleux A, Lefebvre P et al (2017) Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152(7):1679-1694.e3
Kazak L, Chouchani ET, Lu GZ et al (2017) Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab 26(4):693
Kazak L, Cohen P (2020) Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat Rev Endocrinol 16(8):421–436
Hammerschmidt P, Ostkotte D, Nolte H et al (2019) CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177(6):1536-1552.e23
Huang T, Song J, Gao J et al (2022) Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat Commun 13(1):3489
Wlodarska M, Luo C, Kolde R et al (2017) Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 22(1):25-37e6
Du J, Xi L, Zhang Z et al (2023) Metabolic remodeling of glycerophospholipids acts as a signature of dulaglutide and liraglutide treatment in recent-onset type 2 diabetes mellitus. Front Endocrinol 13:1097612
Misselbeck K, Parolo S, Lorenzini F et al (2019) A network-based approach to identify deregulated pathways and drug effects in metabolic syndrome. Nat Commun 10(1):5215
Qin D, Lei Y, Xie W et al (2023) Methionine sulfoxide suppresses adipogenic differentiation by regulating the mitogen-activated protein kinase signaling pathway. Cell Biol Int 47:648–649
Gao J, Xu K, Liu H et al (2018) Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 8:13
Lin J, Sun-Waterhouse D, Cui C (2022) The therapeutic potential of diet on immune-related diseases: based on the regulation on tryptophan metabolism. Crit Rev Food Sci Nutr 62(31):8793–8811
Sun P, Wang M, Li Z et al (2022) Eucommiae cortex polysaccharides mitigate obesogenic diet-induced cognitive and social dysfunction via modulation of gut microbiota and tryptophan metabolism. Theranostics 12(8):3637–3655
Agus A, Clement K, Sokol H (2021) Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70(6):1174–1182
Wu Z, Du Z, Tian Y et al (2022) Inulin accelerates weight loss in obese mice by regulating gut microbiota and serum metabolites. Front Nutr 9:980382
Yue S, Zhao D, Peng C et al (2019) Effects of theabrownin on serum metabolites and gut microbiome in rats with a high-sugar diet. Food Funct 10(11):7063–7080
Shi SY, Luk CT, Brunt JJ et al (2014) Adipocyte-specific deficiency of Janus kinase (JAK) 2 in mice impairs lipolysis and increases body weight, and leads to insulin resistance with ageing. Diabetologia 57(5):1016–1026
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117(Pt 8):1281–1283
Burrell JA, Boudreau A, Stephens JM (2020) Latest advances in STAT signaling and function in adipocytes. Clin Sci (Lond) 134(6):629–639
Lee K, Um SH, Rhee DK et al (2016) Interferon-alpha inhibits adipogenesis via regulation of JAK/STAT1 signaling. Biochim Biophys Acta 1860(Pt 11 A):2416–2427
Acknowledgements
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This project was supported by the National Key Research and Development Program of China (2021YFC2701900, 2021YFC2701903), National Natural Science Foundation of China (No. 82170869, No. 82100915), Natural Science Foundation Project of Shanghai Scientific and technological innovation plan (No.22ZR1457000) and the Master and Doctor innovation talent base for endocrine and metabolic diseases (RCJD2021S03).
Author information
Authors and Affiliations
Contributions
XG, XD, SH, and JG designed the project and edited the manuscript. LZ, JZ, and JG performed the experiment and manuscript preparation. ZP, ZZ, and LZ performed the data analyses and statistics analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
The study involving human participants was approved by the Medical Ethics Committee of Shanghai Tongren Hospital of China (approval No. 2021-059-01). The patients/participants provided their written informed consent to participate in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The experimental procedures of animal were approved by the Medical Ethics Committee of Shanghai Tongren Hospital of China (approval No. A2022-017-01). All experiments were performed in accordance with ARRIVE guidelines and regulations.
Informed consent
All persons gave their informed consent prior to their inclusion in the study.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
592_2024_2256_MOESM1_ESM.jpg
Figure S1. The effect of IA on the early development and survival rate of zebrafish embryos. (A) Mortality rate at 72hpf. (B) Morphology changes at 72hpf. Data were obtained from three experiments (n > 30), and the graph shows the mean ± SD. hpf, hours post fertilization. (JPG 81 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Zhao, J., Peng, Z. et al. Anti-adipogenesis effect of indole-3-acrylic acid on human preadipocytes and HFD-induced zebrafish. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02256-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00592-024-02256-7