Abstract
Aim
Implementing genetic analyses have unraveled rare alterations causing early-onset obesity and complications, in whom treatment is challenging. We aimed to report on the effects of adjuvant off-label therapy with liraglutide, glucagon-like peptide-1 analogue (GLP-1a), in rare genetic diagnoses.
Methods
Case scenarios and review of the literature.
Results
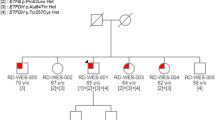
Case 1: Nine-year-old boy with early-onset severe obesity and nonalcoholic fatty liver disease (NAFLD) due to a homozygous mutation in the MC4R gene deteriorated under lifestyle change and metformin therapy [at 10.5 years: body mass index (BMI) 51.2kg/m2, 226% of the 95th percentile, fat percentage (FP) 65% and muscle-to-fat ratio (MFR) z-score of −2.41]. One year of liraglutide treatment halted progressive weight gain [BMI 50.3kg/m2, 212% of the 95th percentile, 63.7% FP and MFR z-score of −2.34], with biochemical improvement.
Case 2: Twelve-year-old boy with obesity presented with diabetes and progressive NAFLD. Exome analysis revealed two heterozygous mutations compatible with monogenic diabetes (HNF1A) and familial hypercholesterolemia (LDLR). Lifestyle modifications resulted in clinical and laboratory improvement (BMI 87th percentile, 32.8% FP, MFR z-score of −1.63, HbA1c 5.5%) without the expected recovery in liver transaminases. Liraglutide treatment augmented the improvement in weight status (BMI 68th percentile, 22.6% FP, MFR z-score of −1.13) with normalization of liver transaminases.
Case 3: Nineteen-year-old male with spinal muscular atrophy type 3 presented with sarcopenic obesity and comorbidities. Treatment strategy included dietary counseling and multiple drug therapies (metformin, anti-hypertensive and statins). Liraglutide therapy led to a gradual recovery of metabolic complications allowing tapering-down other medications.
Conclusions
Considering the pleiotropic effects of GLP1-a beyond BMI reduction, this treatment modality may serve as a game changer in challenging cases.

Similar content being viewed by others
Data availability
All data are provided in the manuscript.
References
Jebeile H, Kelly AS, O’Malley G, Baur LA (2022) Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol 10:351–365. https://doi.org/10.1016/S2213-8587(22)00047-X
Serra-Juhé C, Martos-Moreno G, Bou de Pieri F et al (2020) Heterozygous rare genetic variants in non-syndromic early-onset obesity. Int J Obes (Lond) 44:830–841. https://doi.org/10.1038/S41366-019-0357-5
Loos RJF, Yeo GSH (2022) The genetics of obesity: from discovery to biology. Nat Rev Genet 23:120–133. https://doi.org/10.1038/S41576-021-00414-Z
Thaker VV (2017) Genetic and epigenetic causes of obesity. Adolesc Med State Art Rev 28:379–405. https://doi.org/10.1542/9781581109405-genetic
Farooqi IS (2021) Monogenic human obesity syndromes. Handb Clin Neurol 181:301–310. https://doi.org/10.1016/B978-0-12-820683-6.00022-1
Liu F, Zhu X, Jiang X et al (2022) Transcriptional control by HNF-1: emerging evidence showing its role in lipid metabolism and lipid metabolism disorders. Genes Dis 9:1248. https://doi.org/10.1016/J.GENDIS.2021.06.010
Brener A, Lebenthal Y, Shtamler A et al (2020) The endocrine manifestations of spinal muscular atrophy, a real-life observational study. Neuromuscul Disord 30:270–276. https://doi.org/10.1016/J.NMD.2020.02.011
Smith NK, Hackett TA, Galli A, Flynn CR (2019) GLP-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem Int 128:94. https://doi.org/10.1016/J.NEUINT.2019.04.010
Müller TD, Finan B, Bloom SR et al (2019) Glucagon-like peptide 1 (GLP-1). Mol Metab 30:72–130. https://doi.org/10.1016/J.MOLMET.2019.09.010
Spencer NJ, Hibberd TJ (2022) GLP-1 appetite control via intestinofugal neurons. Cell Res. https://doi.org/10.1038/S41422-022-00692-0
Holst JJ (2019) The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism 96:46–55. https://doi.org/10.1016/J.METABOL.2019.04.014
Gasbjerg LS, Bergmann NC, Stensen S et al (2020) Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides. https://doi.org/10.1016/J.PEPTIDES.2019.170183
Ma X, Liu Z, Ilyas I et al (2021) GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci 17:2050–2068. https://doi.org/10.7150/IJBS.59965
Andrikou E, Tsioufis C, Andrikou I et al (2019) GLP-1 receptor agonists and cardiovascular outcome trials: an update. Hellenic J Cardiol 60:347–351. https://doi.org/10.1016/J.HJC.2018.11.008
Basalay MV, Mastitskaya S, Mrochek A et al (2016) Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res 112:669–676. https://doi.org/10.1093/CVR/CVW216
Talbot EA, Brown KH, Kirkland KB et al (2010) The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak. Vaccine 28:8001–8007. https://doi.org/10.1016/j.vaccine.2010.09.034
Hira T, Pinyo J, Hara H (2020) What Is GLP-1 really doing in obesity? Trends Endocrinol Metab 31:71–80. https://doi.org/10.1016/J.TEM.2019.09.003
Gilbert MP, Pratley RE (2020) GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol (Lausanne). https://doi.org/10.3389/FENDO.2020.00178
Zhao X, Wang M, Wen Z et al (2021) GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne). https://doi.org/10.3389/FENDO.2021.721135
Aroda VR (2018) A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 20(Suppl 1):22–33. https://doi.org/10.1111/DOM.13162
Kelly AS, Auerbach P, Barrientos-Perez M et al (2020) A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 382:2117–2128. https://doi.org/10.1056/NEJMOA1916038
Ryan PM, Seltzer S, Hayward NE et al (2021) Safety and efficacy of glucagon-like peptide-1 receptor agonists in children and adolescents with obesity: a meta-analysis. J Pediatr 236:137-147.e13. https://doi.org/10.1016/J.JPEDS.2021.05.009
FDA approves weight management drug for patients aged 12 and older | FDA. In: U.S. FOOD DRUG Adm. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older. Accessed 18 Oct 2022
Brener A, Peleg I, Rosenfeld T et al (2021) Beyond body mass index - body composition assessment by bioimpedance in routine endocrine practice. Endocr Pract 27:419–425. https://doi.org/10.1016/J.EPRAC.2020.10.013
McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM (2014) Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes 9:249–259. https://doi.org/10.1111/J.2047-6310.2013.00168.X
Matthews DR, Hosker JP, Rudenski AS et al (1985) (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetol 287(28):412–419. https://doi.org/10.1007/BF00280883
Daniels SR, Greer FR (2008) Lipid screening and cardiovascular health in childhood. Pediatrics. https://doi.org/10.1542/peds.2008-1349
Nur ZatiIwani AK, Jalaludin MY, Yahya A et al (2022) TG: HDL-C ratio as insulin resistance marker for metabolic syndrome in children with obesity. Front Endocrinol (Lausanne). https://doi.org/10.3389/FENDO.2022.852290
Newsome PN, Sasso M, Deeks JJ et al (2020) FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 5:362–373. https://doi.org/10.1016/S2468-1253(19)30383-8
VCV000435828.8 - ClinVar - NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/435828/?new_evidence=true. Accessed 8 Nov 2022
Grinbaum R, Beglaibter N, Mitrani-Rosenbaum S et al (2022) The Obesogenic and glycemic effect of bariatric surgery in a family with a melanocortin 4 receptor loss-of-function mutation. Metabolites 12:430. https://doi.org/10.3390/METABO12050430
Maturity-Onset Diabetes of the Young, Type 3 disease: Malacards - Research Articles, Drugs, Genes, Clinical Trials. https://www.malacards.org/card/maturity_onset_diabetes_of_the_young_type_3?showAll=True. Accessed 8 Nov 2022
NM_000527.5(LDLR):c.1720C>T (p.Arg574Cys) AND Hypercholesterolemia, familial, 1 - ClinVar - NCBI. https://www.ncbi.nlm.nih.gov/clinvar/RCV000238063/. Accessed 8 Nov 2022
Newsome PN, Buchholtz K, Cusi K et al (2021) A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 384:1113–1124. https://doi.org/10.1056/NEJMOA2028395
Ceriotti F, Henny J, Queraltó J et al (2010) Common reference intervals for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transferase (GGT) in serum: results from an IFCC multicenter study. Clin Chem Lab Med 48:1593–1601. https://doi.org/10.1515/CCLM.2010.315/MACHINEREADABLECITATION/RIS
Kwo PY, Cohen SM, Lim JK (2017) ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 112:18–35. https://doi.org/10.1038/AJG.2016.517
Iepsen EW, Have CT, Veedfald S et al (2020) GLP-1 receptor agonist treatment in morbid obesity and type 2 diabetes due to pathogenic homozygous melanocortin-4 receptor mutation: a case report. Cell reports Med. https://doi.org/10.1016/J.XCRM.2020.100006
Bae JH, Choi HJ, Cho KIK et al (2020) Glucagon-like peptide-1 receptor agonist differentially affects brain activation in response to visual food cues in lean and obese individuals with type 2 diabetes mellitus. Diabetes Metab J 44:248. https://doi.org/10.4093/DMJ.2019.0018
Østoft SH, Bagger JI, Hansen T et al (2014) Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care 37:1797–1805. https://doi.org/10.2337/DC13-3007
Mantovani A, Petracca G, Beatrice G et al (2021) Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites 11:1–13. https://doi.org/10.3390/METABO11020073
Wu L, Zhou M, Li T et al (2022) GLP-1 regulates exercise endurance and skeletal muscle remodeling via GLP-1R/AMPK pathway. Biochim Biophys acta Mol cell Res. https://doi.org/10.1016/J.BBAMCR.2022.119300
Yamada S, Ogura Y, Inoue K et al (2022) Effect of GLP-1 receptor agonist, liraglutide, on muscle in spontaneously diabetic torii fatty rats. Mol Cell Endocrinol 539:111472. https://doi.org/10.1016/J.MCE.2021.111472
Abdulla H, Phillips BE, Wilkinson DJ et al (2020) Glucagon-like peptide 1 infusions overcome anabolic resistance to feeding in older human muscle. Aging Cell 19:e13202. https://doi.org/10.1111/ACEL.13202
Gurjar AA, Kushwaha S, Chattopadhyay S et al (2020) Long acting GLP-1 analog liraglutide ameliorates skeletal muscle atrophy in rodents. Metabolism 103:154044. https://doi.org/10.1016/J.METABOL.2019.154044
Ayan E, DeMirci H (2022) A brief atlas of insulin. Curr Diabetes Rev. https://doi.org/10.2174/1573399819666220610150342
Acknowledgements
The authors are grateful to the patients and their families for their consent to share their unique vignettes, to the multidisciplinary team of dedicated health-care professionals caring for these patients, and to Esther Eshkol for editorial assistance.
Funding
None.
Author information
Authors and Affiliations
Contributions
YL and AB helped in conceptualization; HZ, RL, AA, HM-L, LS, MY-B, OB, EC, YL, and AB helped in data curation; HZ, RL, AA, HM-L, LS, MY-B, OB, EC, YL, and AB worked in investigation; HZ and AB helped in writing—original draft; and HZ, RL, AA, HM-L, LS, MY-B, OB, EC, YL, and AB helped in writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The institutional ethics committee after careful consideration and deliberation in each case approved the off-label use of liraglutide.
Informed consent
The patients and their parents gave their informed consent to publish their case.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaitoon, H., Lubetzky, R., Amir, A.Z. et al. Glucagon-like peptide-1 analog therapy in rare genetic diseases: monogenic obesity, monogenic diabetes, and spinal muscular atrophy. Acta Diabetol 60, 1099–1108 (2023). https://doi.org/10.1007/s00592-023-02109-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02109-9