Abstract
Aims
Longitudinal data linking non-alcoholic fatty liver disease to kidney dysfunction in type 2 diabetes (T2D) are limited. This study evaluated the associations of non-invasive indices of liver steatosis and liver fibrosis with kidney impairment, and the mediatory role of the pro-angiogenic factor leucine-rich α-2 glycoprotein 1 (LRG1).
Methods
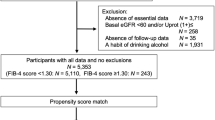
T2D adults (n = 2057) were followed for a mean period of 6.1 ± 1.6 years. Baseline liver steatosis [(hepatic steatosis index (HSI) and Zhejiang University index (ZJU)] and liver fibrosis [aspartate transaminase/alanine transaminase ratio (AAR) and BARD] indices derived from composite scoring systems were calculated. Plasma LRG1 levels were quantified using immunoassay. The study outcomes were progressive kidney function decline defined as estimated glomerular filtration rate (eGFR) decline of ≥ 40% and albuminuria progression defined as an increase in albuminuria category.
Results
Cross-sectionally, liver steatosis and liver fibrosis indices were associated with increased albuminuria (urinary albumin/creatinine ratio ≥ 30 µg/mg) and reduced renal function (eGFR < 60 mL/min/1.73 m2) after covariate adjustment, respectively. Approximately 32% of the participants experienced progressive kidney function decline, while 38% had albuminuria worsening over time. Longitudinal analysis revealed that baseline AAR (hazard ratio: 1.56; 95% CI 1.15–2.11) and BARD (hazard ratio: 1.16, 95% CI 1.04–1.28) predicted progressive kidney function decline, partly mediated by LRG1. In contrast, liver steatosis (HSI and ZJU) but not liver fibrosis (AAR and BARD) indices were independently associated with albuminuria progression.
Conclusions
Increased liver steatosis scores were associated with albuminuria deterioration. Conversely, liver fibrosis indices may be associated with progressive kidney function decline, potentially driven by increased inflammation and angiogenesis.

Similar content being viewed by others
References
Low S, Tai ES, Yeoh LY et al (2016) Onset and progression of kidney disease in type 2 diabetes among multi-ethnic Asian population. J Diabetes Complicat 30:1248–1254. https://doi.org/10.1016/j.jdiacomp.2016.05.020
Mantovani A, Byrne CD, Bonora E, Targher G (2018) Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 41:372–382. https://doi.org/10.2337/dc17-1902
Foschi FG, Conti F, Domenicali M et al (2021) External validation of surrogate indices of fatty liver in the general population: the Bagnacavallo study. J Clin Med 10:520. https://doi.org/10.3390/jcm10030520
Raszeja-Wyszomirska J, Szymanik B, Lawniczak M et al (2010) Validation of the BARD scoring system in polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol 10:67. https://doi.org/10.1186/1471-230X-10-67
Thong VD, Quynh BTH (2021) Correlation of serum transaminase levels with liver fibrosis assessed by transient elastography in vietnamese patients with nonalcoholic fatty liver disease. Int J Gen Med 14:1349–1355. https://doi.org/10.2147/IJGM.S309311
Targher G, Bertolini L, Rodella S et al (2008) Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 51:444–450. https://doi.org/10.1007/s00125-007-0897-4
Ciardullo S, Ballabeni C, Trevisan R, Perseghin G (2022) Liver stiffness, albuminuria and chronic kidney disease in patients with NAFLD: a systematic review and meta-analysis. Biomolecules 12:105. https://doi.org/10.3390/biom12010105
Targher G, Chonchol M, Bertolini L et al (2008) Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 19:1564–1570. https://doi.org/10.1681/ASN.2007101155
Mantovani A, Petracca G, Beatrice G et al (2022) Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut 71:156–162. https://doi.org/10.1136/gutjnl-2020-323082
Jia G, Di F, Wang Q et al (2015) Non-alcoholic fatty liver disease is a risk factor for the development of diabetic nephropathy in patients with type 2 diabetes mellitus. PLoS ONE 10:e0142808. https://doi.org/10.1371/journal.pone.0142808
Lee YJ, Wang CP, Hung WC et al (2020) Common and unique factors and the bidirectional relationship between chronic kidney disease and nonalcoholic fatty liver in type 2 diabetes patients. Diabetes Metab Syndr Obes 13:1203–1214. https://doi.org/10.2147/DMSO.S237700
Meng H, Song Y, Zhu J et al (2016) LRG1 promotes angiogenesis through upregulating the TGFbeta1 pathway in ischemic rat brain. Mol Med Rep 14:5535–5543. https://doi.org/10.3892/mmr.2016.5925
O’Donnell LC, Druhan LJ, Avalos BR (2002) Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol 72:478–485
Liu JJ, Pek SLT, Ang K, Tavintharan S, Lim SC, Study SD (2017) Plasma leucine-rich alpha-2-glycoprotein 1 predicts rapid eGFR Decline and albuminuria progression in type 2 diabetes mellitus. J Clin Endocrinol Metab 102:3683–3691. https://doi.org/10.1210/jc.2017-00930
Pek SL, Tavintharan S, Wang X et al (2015) Elevation of a novel angiogenic factor, leucine-rich-alpha2-glycoprotein (LRG1), is associated with arterial stiffness, endothelial dysfunction, and peripheral arterial disease in patients with type 2 diabetes. J Clin Endocrinol Metab 100:1586–1593. https://doi.org/10.1210/jc.2014-3855
Zhang X, Pek SLT, Tavintharan S et al (2019) Leucine-rich alpha-2-glycoprotein predicts proliferative diabetic retinopathy in type 2 diabetes. J Diabetes Complicat 33:651–656. https://doi.org/10.1016/j.jdiacomp.2019.05.021
Liu JJ, Pek SLT, Wang J et al (2020) Association OF Plasma leucine-rich alpha-2 glycoprotein 1, a modulator of transforming growth factor-beta signaling pathway, with incident heart failure in individuals with type 2 diabetes. Diabetes Care 44:571–577. https://doi.org/10.2337/dc20-2065
Moh MC, Sum CF, Tavintharan S et al (2019) Gain in adiposity over 3 years is associated with progressive renal decline in multi-ethnic South-east Asians with type 2 diabetes. J Diabetes 11:316–325. https://doi.org/10.1111/1753-0407.12848
Cohen JA, Kaplan MM (1979) The SGOT/SGPT ratio: an indicator of alcoholic liver disease. Dig Dis Sci 24:835–838. https://doi.org/10.1007/BF01324898
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Levey AS, Gansevoort RT, Coresh J et al (2020) Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration with the US food and drug administration and European medicines agency. Am J Kidney Dis 75:84–104. https://doi.org/10.1053/j.ajkd.2019.06.009
Macisaac RJ, Ekinci EI, Jerums G (2014) Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 63:S39-62. https://doi.org/10.1053/j.ajkd.2013.10.048
Lee JH, Kim D, Kim HJ et al (2010) Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 42:503–508. https://doi.org/10.1016/j.dld.2009.08.002
Wang J, Xu C, Xun Y et al (2015) ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep 5:16494. https://doi.org/10.1038/srep16494
Sorbi D, Boynton J, Lindor KD (1999) The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol 94:1018–1022. https://doi.org/10.1111/j.1572-0241.1999.01006.x
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA (2008) Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 57:1441–1447. https://doi.org/10.1136/gut.2007.146019
Kahl S, Strassburger K, Nowotny B, Livingstone R et al (2014) Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS ONE 9:e94059. https://doi.org/10.1371/journal.pone.0094059
Wang Q, Xie W, Liu L, Wang P, Pan CQ (2021) Serum markers for predicting advanced fibrosis in patients with chronic hepatitis B and nonalcoholic fatty liver disease. Medicine (Baltimore) 100:e25327. https://doi.org/10.1097/MD.0000000000025327
Ciardullo S, Muraca E, Perra S et al (2020) Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care 8:e000904. https://doi.org/10.1136/bmjdrc-2019-000904
Han E, Cho Y, Kim KW et al (2020) Hepatic fibrosis is associated with total proteinuria in Korean patients with type 2 diabetes. Medicine (Baltimore) 99:e21038. https://doi.org/10.1097/MD.0000000000021038
Seo DH, Suh YJ, Cho Y et al (2022) Advanced liver fibrosis is associated with chronic kidney disease in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Diabetes Metab J 46:630–639. https://doi.org/10.4093/dmj.2021.0130
Liu JJ, Ghosh S, Kovalik JP et al (2017) Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney Int Rep 2:470–480. https://doi.org/10.1016/j.ekir.2016.12.003
Yeung MW, Wong GL, Choi KC et al (2017) Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J Hepatol 68:147–156. https://doi.org/10.1016/j.jhep.2017.09.020
Weil EJ, Lemley KV, Mason CC et al (2012) Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 82:1010–1017. https://doi.org/10.1038/ki.2012.234
Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89:1221–1230. https://doi.org/10.1016/j.kint.2016.01.012
Ray L, Nanda SK, Chatterjee A, Sarangi R, Ganguly S (2015) A comparative study of serum aminotransferases in chronic kidney disease with and without end-stage renal disease: need for new reference ranges. Int J Appl Basic Med Res 5:31–35. https://doi.org/10.4103/2229-516X.149232
Sette LH, Lopes EP (2015) The reduction of serum aminotransferase levels is proportional to the decline of the glomerular filtration rate in patients with chronic kidney disease. Clinics (Sao Paulo) 70:346–349. https://doi.org/10.6061/clinics/2015(05)07
Ochiai H, Shirasawa T, Yoshimoto T et al (2020) Elevated alanine aminotransferase and low aspartate aminotransferase/alanine aminotransferase ratio are associated with chronic kidney disease among middle-aged women: a cross-sectional study. BMC Nephrol 21:471. https://doi.org/10.1186/s12882-020-02144-6
Missikpode C, Kramer H, Cotler SJ et al (2021) Association of elevated serum aminotransferase levels with chronic kidney disease measures: hispanic community health study/study of latinos. BMC Nephrol 22:302. https://doi.org/10.1186/s12882-021-02483-y
Acknowledgements
The authors thank the staff from the Singapore Clinical Research Institute (SCRI) for their contribution to the study protocol and database design.
Funding
This study was supported by the Alexandra Health Fund Ltd through the Science—Translational and Applied Research Grants STAR17202 and STAR19103. The SMART2D cohort was supported by the Singapore Ministry of Health's National Medical Research Council CS-IRG (MOH-000066). Su Chi Lim was supported by the Singapore Ministry of Health's National Medical Research Council NMRC/CSA-INV/0020/2017, MOH-000066, and MOH-0000714-01.
Author information
Authors and Affiliations
Contributions
MCM analysed the data and drafted the manuscript. TS, WET, SBML, CFS, and SCL contributed to the conception and design of the study. KCPS, SLTP, SL, and KA acquired and/or interpreted the data. SCL is the guarantor of the manuscript. All authors critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval, Human and Animal Rights
The study was performed in accordance with the 1964 Helsinki Declaration and its later amendments and was approved by the National Healthcare Group Domain Specific Ethics Review Board [reference numbers: 2017/00561 and 2019/00693].
Consent to participate, Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moh, M.C., Pek, S.L.T., Sze, K.C.P. et al. Associations of non-invasive indices of liver steatosis and fibrosis with progressive kidney impairment in adults with type 2 diabetes. Acta Diabetol 60, 827–835 (2023). https://doi.org/10.1007/s00592-023-02058-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02058-3