Abstract
Aims
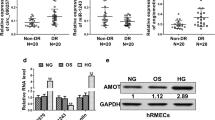
Diabetes retinopathy (DR) is associated with retinal microvascular system injury induced by high glucose (HG). This study aims to explore the role and mechanism of long non-coding RNA THRIL in regulating cell proliferation and migration of human retina microvascular endothelial cells (hRMECs) under HG condition.
Method
The gene and protein expression were detetced by RT-PCR and western blot, respectively. Cell proliferation and migration of hRMECs were examined using MTT assay and Transwell assay, respectively. The interaction between miR-125b-5p and THRIL or autophagy-related gene 4D (ATG4D) was analyzed using luciferase activity assay.
Results
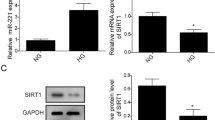
THRIL expression was induced by HG in hRMECs. THRIL overexpression enhanced the proliferation and migration of hRMECs induced by HG, whereas THRIL silencing yielded the opposite results. Furthermore, THRIL overexpression induced autophagy activation, and inhibition of autophagy by 3-methyladenine abrogated the promotory effects of THRIL overexpression on cell proliferation and migration of hRMECs. Mechanismly, THRIL inhibited miR-125b-5p to upregulate the expression of ATG4D (an important autophagy-related gene), thereby promoting autophagy. Moreover, miR-125b-5p overexpression or ATG4D silencing alone abolished the promoting effects of THRIL overexpression on HG-induced autophagy, proliferation and migration of hRMECs.
Conclusions
THRIL promotes HG-induced cell proliferation and migration of hRMECs through activation of autophagy via the miR-125b-5p/ATG4D axis. THRIL may serve as a potential therapeutic target for DR.





Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Whitehead M, Wickremasinghe S, Osborne A, Van Wijngaarden P, Martin KR (2018) Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Exp Opin Biol Ther 18(12):1257–1270. https://doi.org/10.1080/14712598.2018.1545836
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366(13):1227–1239. https://doi.org/10.1056/NEJMra1005073
Dehdashtian E, Mehrzadi S, Yousefi B et al. (2018) Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci 193:20–33. https://doi.org/10.1016/j.lfs.2017.12.001
Wang Y, Liu X, Zhu L et al. (2020) PG545 alleviates diabetic retinopathy by promoting retinal Müller cell autophagy to inhibit the inflammatory response. Biochem Biophys Res Commun 531(4):452–458. https://doi.org/10.1016/j.bbrc.2020.07.134
Li R, Du J, Yao Y, Yao G, Wang X (2019) Adiponectin inhibits high glucose-induced angiogenesis via inhibiting autophagy in RF/6A cells. J Cell Physiol 234(11):20566–20576. https://doi.org/10.1002/jcp.28659
Cai X, Li J, Wang M et al (2017) GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int J Med Sci 14(12):1203–1212. https://doi.org/10.7150/ijms.20962
Biswas S, Sarabusky M, Chakrabarti S (2019) Diabetic retinopathy, lncRNAs, and inflammation: a dynamic, interconnected network. J Clin Med 8(7):1033. https://doi.org/10.3390/jcm8071033
Zhang J, Chen M, Chen J et al. (2017) Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Biosci Rep 37 (2):BSR20170036. 10.1042/bsr20170036
Thomas AA, Feng B, Chakrabarti S (2017) ANRIL: a regulator of VEGF in diabetic retinopathy. Invest Ophthalmol Vis Sci 58(1):470–480. https://doi.org/10.1167/iovs.16-20569
Li Z, Chao TC, Chang KY et al. (2014) The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proceed Natl Acad Sci USA 111(3):1002–1007. https://doi.org/10.1073/pnas.1313768111
Xu B, Jin X, Yang T et al. (2020) Upregulated lncRNA THRIL/TNF-α signals promote cell growth and predict poor clinical outcomes of osteosarcoma. OncoTargets Ther 13:119–129. https://doi.org/10.2147/ott.s235798
Xiao J, Lu Y, Yang X (2020) THRIL mediates endothelial progenitor cells autophagy via AKT pathway and FUS. Mol Med 26(1):86. https://doi.org/10.1186/s10020-020-00201-2
Li X, Yu ZW, Wang Y, Fu YH, Gao XY (2020) MicroRNAs: potential targets in diabetic retinopathy. Horm Metab Res 52(3):142–148. https://doi.org/10.1055/a-1107-2943
Ye Z, Li ZH, He SZ (2017) miRNA-1273g-3p Involvement in development of diabetic retinopathy by modulating the autophagy-lysosome pathway. Med Sci Monit 23:5744–5751. https://doi.org/10.12659/msm.905336
Zhong X, Chen O, Zhou T, Lü M, Wan J (2021) Cytotoxin-associated gene A-positive Helicobacter pylori promotes autophagy in colon cancer cells by inhibiting miR-125b-5p. Can J Infect Dis Med Microbiol 2021:6622092. https://doi.org/10.1155/2021/6622092
Liu G, Wan Q, Li J, Hu X, Gu X, Xu S (2020) Silencing miR-125b-5p attenuates inflammatory response and apoptosis inhibition in mycobacterium tuberculosis-infected human macrophages by targeting DNA damage-regulated autophagy modulator 2 (DRAM2). Cell Cycle 19(22):3182–3194. https://doi.org/10.1080/15384101.2020.1838792
Fan Y, Zhao X, Lu K, Cheng G (2020) LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson’s disease via ablating microRNA-125b-5p. Brain Res Bull 157:119–127. https://doi.org/10.1016/j.brainresbull.2020.02.003
Qiu J, Zhu J, Zhang R et al. (2019) miR-125b-5p targeting TRAF6 relieves skeletal muscle atrophy induced by fasting or denervation. Ann Transl Med 7 (18):456. https://doi.org/10.21037/atm.2019.08.39
Gong Q, Xie J, Li Y, Liu Y, Su G (2019) Enhanced ROBO4 is mediated by up-regulation of HIF-1α/SP1 or reduction in miR-125b-5p/miR-146a-5p in diabetic retinopathy. J Cell Mol Med 23(7):4723–4737. https://doi.org/10.1111/jcmm.14369
Liu G, Wang Y, Zhang M, Zhang Q (2019) Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol 66:354–361. https://doi.org/10.1016/j.intimp.2018.11.038
Russo R, Varano GP, Adornetto A et al (2018) Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis 9(10):981. https://doi.org/10.1038/s41419-018-1044-5
Betin VM, Lane JD (2009) Atg4D at the interface between autophagy and apoptosis. Autophagy 5(7):1057–1059. https://doi.org/10.4161/auto.5.7.9684
Zhao JY, Li XY, Liu TD, Liang B, Huang Y, Li W (2021) Silencing of ATG4D suppressed proliferation and enhanced cisplatin-induced apoptosis in hepatocellular carcinoma through Akt/Caspase-3 pathway. Mol Cell Biochem. https://doi.org/10.1007/s11010-021-04224-z
Liu P, Jia SB, Shi JM et al (2019) LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci Rep 39 (5). 10.1042/bsr20181469
Hammes HP, Feng Y, Pfister F, Brownlee M (2011) Diabetic retinopathy: targeting vasoregression. Diabetes 60(1):9–16. https://doi.org/10.2337/db10-0454
Biswas S, Thomas AA, Chen S et al (2018) MALAT1: an epigenetic regulator of inflammation in diabetic retinopathy. Sci Rep 8(1):6526. https://doi.org/10.1038/s41598-018-24907-w
Thomas AA, Biswas S, Feng B, Chen S, Gonder J, Chakrabarti S (2019) lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia 62(3):517–530. https://doi.org/10.1007/s00125-018-4797-6
Tong P, Peng QH, Gu LM, Xie WW, Li WJ (2019) LncRNA-MEG3 alleviates high glucose induced inflammation and apoptosis of retina epithelial cells via regulating miR-34a/SIRT1 axis. Exp Mol Pathol 107:102–109. https://doi.org/10.1016/j.yexmp.2018.12.003
Liu T, Liu J, Tian C, Wang H, Wen M, Yan M (2020) LncRNA THRIL is upregulated in sepsis and sponges miR-19a to upregulate TNF-α in human bronchial epithelial cells. J Inflammation 17:31. https://doi.org/10.1186/s12950-020-00259-z
Chen H, Hu X, Li R et al (2020) LncRNA THRIL aggravates sepsis-induced acute lung injury by regulating miR-424/ROCK2 axis. Mol Immunol 126:111–119. https://doi.org/10.1016/j.molimm.2020.07.021
Ayoub SE, Hefzy EM, Abd El-Hmid RG et al (2020) Analysis of the expression profile of long non-coding RNAs MALAT1 and THRIL in children with immune thrombocytopenia. IUBMB Life. https://doi.org/10.1002/iub.2310
Liang Y, Li H, Gong X, Ding C (2020) Long non-coding RNA THRIL mediates cell growth and inflammatory response of fibroblast-like synoviocytes by activating PI3K/AKT signals in rheumatoid arthritis. Inflammation 43(3):1044–1053. https://doi.org/10.1007/s10753-020-01189-x
Qi H, Shen J, Zhou W (2020) Up-regulation of long non-coding RNA THRIL in coronary heart disease: prediction for disease risk, correlation with inflammation, coronary artery stenosis, and major adverse cardiovascular events. J Clin Lab Anal 34(5):e23196. https://doi.org/10.1002/jcla.23196
Qiu F, Tong H, Wang Y, Tao J, Wang H, Chen L (2018) Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci Biotechnol Biochem 82(8):1366–1376. https://doi.org/10.1080/09168451.2018.1459179
Zhou P, Xie W, Meng X et al (2019) Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells 8 (3). https://doi.org/10.3390/cells8030213
Lu MH, Tang B, Zeng S et al. (2016) Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene 35(27):3524–3534. https://doi.org/10.1038/onc.2015.413
Cesana M, Cacchiarelli D, Legnini I et al (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147(2):358–369. https://doi.org/10.1016/j.cell.2011.09.028
Deng Y, Luan S, Zhang Q, Xiao Y (2018) Long noncoding RNA THRIL contributes in lipopolysaccharide-induced HK-2 cells injury by sponging miR-34a. J Cell Biochem. https://doi.org/10.1002/jcb.27354
Andaloussi AE, Habib S, Soylemes G et al (2017) Defective expression of ATG4D abrogates autophagy and promotes growth in human uterine fibroids. Cell Death Discov 3:17041. https://doi.org/10.1038/cddiscovery.2017.41
Xu Y, An Y, Wang Y et al (2013) miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep 29(5):2019–2024. https://doi.org/10.3892/or.2013.2338
Acknowledgements
Not applicable.
Funding
This study was supported by grants from Natural Science Foundation of Anhui (1908085MH253).
Author information
Authors and Affiliations
Contributions
QJ, JH designed the study; all authors participated in the experiments; QJ, JH, LS contributed the data analysis; QJ drafted the paper; JH revised the paper. All authors approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The First Affiliated Hospital of USTC.
Consent to publish
Not applicable.
Informed consent
No human samples were involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingshan Ji and Jing Han are co-first authors.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
About this article
Cite this article
Ji, Q., Han, J., Liu, J. et al. LncRNA THRIL promotes high glucose-induced proliferation and migration of human retina microvascular endothelial cells through enhancing autophagy. Acta Diabetol 59, 369–380 (2022). https://doi.org/10.1007/s00592-021-01813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01813-8